FDA Perspective on Epidemiological Cut-off Values (ECVs)
The FDA presents insights on the development and use of Epidemiological Cut-off Values (ECVs) to distinguish wild-type populations from those with acquired resistance mechanisms. ECVs are crucial for determining antimicrobial susceptibility and guiding treatment decisions. The process involves analyzing microbiological data, clinical breakpoints, PK/PD relationships, and clinical trial outcomes. Development programs aim to address unmet needs by providing evidence of in vitro and in vivo activity, PK-PD data, dosage adjustments, safety information, and clinical outcomes in special populations.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
FDA Perspective on the development and use of Epidemiological cut-off values (ECVs) Simone M. Shurland, Ph.D., Division of Anti-Infective Products Office of Antimicrobial Products Office of New Drugs Center for Drug Evaluation and Research Food and Drug Administration
Disclosures None www.fda.gov 2
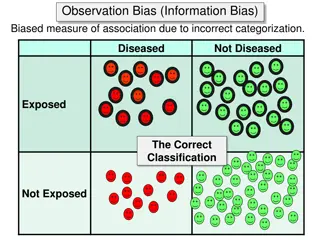
Epidemiological Cut-off Values Epidemiological cut-off value distinguishes wild-type population from those with acquired or selected resistance mechanisms. Wild type are strains that harbor no acquired resistance mechanism, specifically no resistance attributable to Mutation (target alteration) Enzymatic inactivation Up-regulation of efflux pump Up-regulation of target production Metabolic bypass Any combination of the above www.fda.gov 3
Clinical Breakpoint A classification based on an in vitro response of an organism (MIC or zone diameter) to an antimicrobial drug at concentrations corresponding to blood or tissue levels usually attainable at the site of infection with a prescribed labeled dosing regimen. Antimicrobial susceptibility test interpretive criteria are provided to healthcare providers as Susceptible Intermediate Resistant www.fda.gov 4
Data used to derive ECVs v. CB ECVs Microbiological In vitro data (MIC distributions and wild type cut-offs) In vitro resistance markers (both phenotypic and genotypic) Clinical Breakpoints (CB) Microbiological In vitro data (MIC distributions and wild type cut-offs) In vitro resistance markers (both phenotypic and genotypic) Pharmacokinetic/ Pharmacodynamic (PK/PD) Evaluation of PK/PD relationships from animal models of infection Clinical Trial Microbiologic and/or Clinical Outcome by MIC Asin-Prieto et al., J. Infect Chemo 2015 www.fda.gov 5
Development programs to address Unmet Need Data expected in such programs Evidence of in vitro activity and activity in relevant animal models of infection Human PK-PD data Establish MIC breakpoints supported by PK exposure Confirm appropriateness of dosage adjustments/exposures in relevant and special populations Human safety information and non-clinical safety data Smaller clinical datasets with less clinical data likely to be available www.fda.gov 6
ECVs Pros and Cons Examples of uses of microbiological distributions Ideal Splitting the Wildtype Non-Wildtype Population www.fda.gov 7
Example 1 Ideal ECV Distribution www.fda.gov 8
Example 2 - Splitting the Wild Type Population www.fda.gov 9
Example 2 Importance of Non-Wild Type Distribution Probability of Target Attainment Microbiological Eradication Clinical Cure 6000 MIC 5000 N u m b e r o f is o la te s 0.06 98% 98% 100% 4000 0.12 92% 100% 100% 3000 0.25 100%* 100%* 100% 800 0.5 100%* 100%* 100% 600 1 -- -- 100% 400 2 -- -- 100% 200 4 -- -- 100% 0 8 -- -- 100% <0.03 0.03 0.06 0.12 0.25 0.5 1 2 4 8 16 32 >32 16 -- -- 94% M IC 32* 100%* 100%* 39% W ild Type Population Non-W ild Type population www.fda.gov 10
Perspective on Potential Concerns Potential Concern Perspective Some genotypic resistance may not be expressed phenotypically >1 mutation may be required before resistance is expressed clinically or in the laboratory Not all molecular mechanisms responsible for antimicrobial resistance are known Detection and Characterization of Resistance Mechanism Clinical Significance Small shifts upward/downward in MICs may not manifest clinically Downgrade the potential of good drugs Overuse of reserve antibacterial drugs Establishing Susceptibility Criteria for Drugs Developed to Treat Resistant Infections The use of ECVs may be limited in establishing breakpoints for drugs designed to overcome resistance mechanisms www.fda.gov 11
Summary ECVs and CBs have different potential uses. Establishing CBs Microbiologic distribution data is one of the data elements Supportive evidence of other data elements (for instance PK/PD, animal studies) especially in developmental programs with limited clinical data Establishing putative ECVs based on microbiological distribution data will pose challenges. www.fda.gov 12