Innovative Strategies for Building a Strong Cell Therapy Team
Explore strategies shared in the featured sessions from the ISCT Laboratory Practices Committee at the ISCT 2023 North America Regional Meeting, including workforce development, future trends in stem cell processing, and optimizing operations in cell therapy facilities. Learn about effective recruitment, training, and career advancement pathways for cell therapy staff to ensure sustainable growth and success in the field.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
LPC Snapshot: Featured Sessions from the ISCT Laboratory Practices Committee at the ISCT 2023 North America Regional Meeting Ashley A. Krull, Ph.D. Associate Director, Cell Therapy Manufacturing & Engineering Assistant Professor, Hematology The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute
Snapshot Ideas Welcome! The LPC wants Snapshot content to be as helpful and relevant as possible. If you have an idea for a topic you d like to see covered in a Snapshot, please contact us: LPC Chairs Yossi Schwartz (Yossi.Schwartz@moffitt.org) Maggie DiGuardo (DiGuardo.Margaret@mayo.edu) LPC-Telegraft Liaison Ashley Krull (Ashley.Krull@osumc.edu) Post on the ISCT Laboratory Lifeline Community Page 2
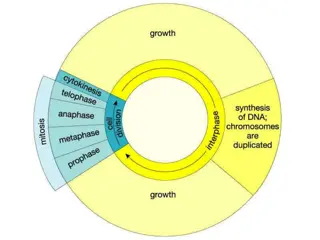
Overview of Sessions Title Moderator, Speakers Elena Maryamchik, MD, MBA Maggie DiGuardo, MD Jelena Holovati, PhD Lauren McVoy, MD, PhD Ashley Krull, PhD Thriving Workforce, Thriving Labs: Strategies for Building Your CGT Team Joseph Schwartz, MD, MPH Cheryl Cox, MHA, MT(ASCP) Jelena Holovati, PhD Events in the CTL The Quadruple C Cryopreservation, Citations, Contamination, and CAPA Joseph Schwartz, MD, MPH Yara Park, MD Lee Buckler, JD Hope Guidry-Groves, BSc Current vs. Future: A Sustainable Business Model for Collection Facilities Elena Maryamchik, MD, MBA Emily Hopewell, PhD Yongping Wang, MD, PhD Karen Moniz, MHA Game-changers for Optimizing Operations at Cell Therapy Processing Facilities Ashley Krull, PhD Melba Marie Page, PhD Brigitte Senechal, PharmD, PhD Scott Jones, PhD Release Assay Qualification & Validation 101 Olive Sturtevant, MHP, MT(ASCP) Sarah Nikiforow, MD, PhD Don Siegel, MD, PhD Tim Wiltshire, PhD ABCs of Point of Care Manufacturing of IEC Maggie DiGuardo, MD Julie Annis, MLS(ASCP) Kathryn Bushnell, MSHS, MLS(ASCP) Wesley McKinney, BSc Transformative Trends: Navigating the Evolution of Stem Cell Processing Labs 3
Thriving Workforce, Thriving Labs: Strategies for Building Your CGT Team Discussed the importance of planning ahead for growth Emphasized advocating for the needs of the staff early and repeatedly Advised working together with the other stakeholders (i.e., clinicians) to get fair reimbursement, adequate staffing and sustainable working conditions. Discussed different pathways for recruiting and training all levels of cell therapy staff, from technicians to lab directors. In-house training programs provide a viable alternative to the traditional educational programs and allow learners to focus on the specifics of cell therapy. Highlighted the need for career ladders for the cell therapy staff, and shared the solutions implemented at the institutions represented. 4
Events in the CTL The Quadruple C Cryopreservation, Citations, Contamination, and CAPA Events happen to everyone better to catch them than never hear about anything! Need to address based on the nature of the event. CAPA is usually required and helpful to prevent recurrence. Stability program (lack of and/or lack of appropriate potency assays) is the most common citation for processing labs based on the 7th edition of the FACT- JACIE standards. 5
Current vs. Future: A Sustainable Business Model for Collection Facilities There is much variation in the collection of MNCs for further manufacturing. Standardization is needed. Collection is a process. Starting material is a product. Consider future issues around access to collection slots. Different models could be used to expand access such as mobile apheresis units and standalone collection centers. 6
Game-changers for Optimizing Operations at Cell Therapy Processing Facilities Highlighted common pain points in growing a cell therapy lab (staffing, training, schedules, implementing new procedures, regulatory and quality issues, and physical facilities) Focus on the culture of flexibility within the lab. Have staff own opportunities for improvement. Remember your Why Advocate for your lab; without them, transplants and therapies don t happen. Partner with MBA students for novel solutions. For complex/rare procedures, better to have designated subject matter experts who can maintain competencies. 7
Release Assay Qualification & Validation 101 Concept of an assay life cycle, one component being assay maintenance, which gets overlooked the most. Maintenance can be achieved by post-implementation monitoring of the assay performance via PT, QC, and equipment comparison. Optimization vs Qualification vs Validation Assay qualification is assay-centric. Assay validation is product-centric. Successful assay validation includes accuracy, precision (repeatability), sensitivity (LOD), specificity (interferences), range, robustness, linearity. Use risk assessment as an analytical control strategy. Common risk factors are reproducibility, vendor stability, and control materials. 8
ABCs of Point of Care Manufacturing of IEC Start with your intended Certificate of Analysis (CoA) and work backwards to plan manufacturing and testing strategy. Use the USP for compendial assay guidance. Consider your infrastructure restraints when optimizing workflow. Good line of communication with clinical care team is crucial. Don t assume what worked for one in- house manufactured product will work for the next, or that the FDA won t ask for new testing. 9
Transformative Trends: Navigating the Evolution of Stem Cell Processing Labs Cross-training evolves, but always maintain 2 subject matter experts. As the lab grows, create specialized positions to give bandwidth back to processing technicians (e.g., education specialists, facilities specialists, inventory manager). Communicate clearly with medical center stakeholders regarding laboratory operations they need to be educated around how you work best. Hire good people who fit and maintain your lab s culture. Bad people can stop progress for entire groups. 10