Bloodborne Pathogens Exposure: HIV Post Exposure Prophylaxis Regimen
Take immediate action for exposure to bloodborne pathogens like HIV and Hepatitis C through a post-exposure prophylaxis (PEP) regimen. Administer PEP within 48 hours for increased-risk exposures like needle sticks or contact with infected fluids. Coordinate evacuation if needed and conduct Rapid Diagnostic Tests. Educate on risk factors and continue monitoring and treatment. Ensure PEP kit contents are properly labeled and include essential items for testing and collection of samples.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Actions to take for exposure to bloodborne pathogens (HIV, Hep C) HIV Post Exposure Prophylaxis Regimen EXPOSURES with increased risk: Needle Stick, Contact with infected or suspected person's fluids, OR contact with assailant body fluid via mucous membrane, non-intact skin or bite; OR unknown exposure (e.g, drug assisted) [AFRICA is considered High Risk] IMMEDIATELY: NO RISK EXPOSURES: Exposures where skin remains intact where there is no anal, vaginal, or oral penetration; no contact with source body fluids via mucous membrane, Begin Soldier Treatment PEP: Delay of PEP administration greater than 48 hours significantly diminishes efficacy of treatment regime. Begin Coordinating for EVAC to Landstuhl: Due to limited PEP supply on hand, evacuation should be completed within 72 hours. Is Source available for RDT (Rapid Diagnostic Test)? No /UNK Is Contact Source HIV Negative? No Use Triple Drug Therapy, currently the best combination: Dolutegravir x 1 tab per day, plus Truvada (two drug combo) X1 Tab per day Pre-treat w/Zofran or Phenergan to offset nausea/vomiting from meds Yes Yes HIV Conduct RDT (Rapid Diagnostic Test) on Source: 1 each, HIV, Hep B, Hep C [do not use saliva, use blood] Is Source RDT Positive? Points of Contact: -LTC Lynch, SOCAF Surgeon (all MEDEVACs) DSN: 314-421-3339 COMM: +49 (0)711-729-3339 SVOIP: (80) 304-436-2035 NIPR: james.h.lynch.mil@mail.mil SIPR: jlynch@usafricom.smil.mil SOCAFRICA JOC: - DSN 421-3340/3370; 0711-729-3340/3370 - RED: 304-436-2100 - socafrica.joc@usafricom.smil.mil usasocafricasocafsurgeon@usafricom.smil.mil ID.consult@us.army.mil AFRICOM ID consultant Submit additional blood sample from potential source to a laboratory for verification. Yes No Blot a sample of Blood onto filter-paper and seal in plastic bag (See Blood Collection and Shipping Instructions) Sample will travel with Soldier during EVAC to Germany and shipped via FedEx using account or be shipped DHL to WRAIR Do you still suspect source HIV positive? Yes No Educate Soldier and unit on Risk Factors and Avoidance Note: Continued Treatment and consultation required, Testing will be required at 1, 3, 6 and 12 months DRAFT
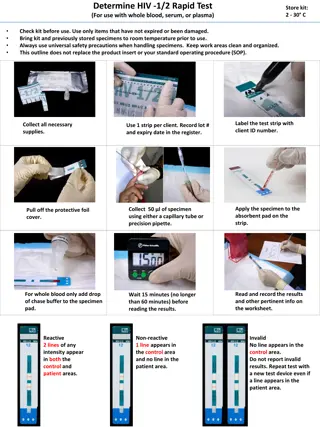
HIV PEP Kit Contents Sealable rugged container: Otter/Pelican approx 5W"x7W"x3D" Labeled on Outside, HIV Exposure Kit Kit Contents with pertinent expiration date Last inspection date and initials Drugs (should medics fill separate in case of expiration date? Dolutegravir Truvada Rapid Test - HIV RDT, (verify no special handling instructions or expiration) Include extra lancet for blood sample Ensure each kit has pipette if applicable (some RDTs have pipets separate) SOP/Flow Chart/shipping instructions - 5x7 laminated printout Blood Sample Kit FTA Micro Card (x2) alcohol wipe sterile lancet adhesive bandage gloves, Multi-Barrier Pouch (strong ziplock to hold the blood sample) mailing envelope tamper-proof tape for the mailing envelope, Desiccant clear outer bag (labeled with code number and expiration date Gloves Pre-Printed labels Desicant bag - keep moisture out
Blood Sample Kit Blood Sample Collection Instructions 5x7 laminated FTA Micro Card (x2) alcohol wipe sterile lancet adhesive bandage gloves, Multi-Barrier Pouch (strong ziplock to hold the blood sample) mailing envelope tamper-proof tape for the mailing envelope, Desiccant clear outer bag (labeled with code number and expiration date
HIV Post Exposure Prophylaxis Regimen Actions to take for exposure to bloodborne pathogens (HIV, Hep C) IMMEDIATELY: HIGH RISK EXPOSURES: Needle Stick, Contact with infected or suspected person's fluids, OR contact with assailant body fluid via mucous membrane, non-intact skin or bite; OR unknown exposure (e.g,, drug assisted) [AFRICA] NO RISK EXPOSURES: No anal, vaginal, or oral penetration; No contact with assailant body fluid via mucous membrane, non-intact skin or bite Contact with fluids of non-infected person Begin Soldier Treatment PEP: Delay of PEP administration greater than 48 hours significantly diminished efficacy of treatment regime. Begin Coordinating for EVAC to Landstuhl: Due to limited PEP supply on hand, evacuation should be completed within 72 hours. Is Contact Source HIV Negative? Is Source available for RDT? No /UNK No Use Triple Drug Therapy, currently the best combination: Daltegravir x 1 tab per day, plus Truvada (two drug combo) X1 Tab per day Pre-treat w/Zofran or Phenergan to offset nausea/vomiting from meds Always submit additional blood sample from the potential source to a laboratory for verification. Blot a sample of Blood onto filter- paper and seal in plastic bag Sample will travel with Soldier during EVAC and turned into lab Yes HIV Conduct RDT on Source Points of Contact: -LTC Lynch, SOCAF Surgeon (all MEDEVACs) DSN: 314-421-3339 COMM: +49 (0)711-729-3339 SVOIP: (80) 304-436-2035 NIPR: james.h.lynch.mil@mail.mil SIPR: jlynch@usafricom.smil.mil Yes Is Source RDT Positive? Yes No ID.consult@us.army.mil AFRICOM ID consultant Educate Soldier and unit on Risk Factors and Avoidance Do you still suspect source HIV positive? No Yes Note: Continued Treatment and consultation required, Testing will be required at 1, 3, 6 and 12 months