Understanding Density and Physical Properties of Matter
Matter possesses physical properties that can be observed without changing its identity, including color, shape, length, mass, volume, and density. Density is the amount of mass per unit volume, where D = m/V. Objects with the same volume but different masses can have varying densities. Density can change with temperature and pressure, as seen in the example of water freezing to ice. Calculating density involves dividing mass by volume. Understanding these concepts is crucial in the study of matter and its properties.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
All matter has physical properties. Matter is anything that has mass and takes up space. Everything that you can see, touch, smell, and taste is matter. A Physical Property is a characteristic of a material that can be observed or measured without changing the identity of the material.
Physical Properties include: Color Shape Length Mass Volume Density What physical properties can you observe in these pictures?
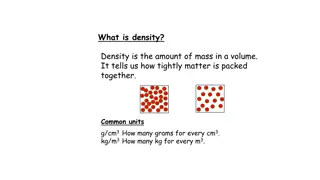
Density Density is the amount of mass a material has for its given volume. Density = mass/volume D=m/V
Same Volume, Different Mass This bowling ball and soccer ball have the same volume, but their masses are different. The bowling ball has more mass per unit volume, so it is more dense.
Temperature and Pressure When the temperature and pressure of a substance changes, the density changes. For example, when liquid water is frozen to ice, the density decreases. Liquid water has a density of 1 g/mL and ice has a density of 0.9168 g/mL
Example Problem A sample of an unknown liquid that has a volume of 70 milliliters weighs 140 grams. What is the density of the liquid? Mass = Volume = D= m/V D=140g/70mL D= 2 g/mL 140g 70mL