Understanding the Role of Phosphatase Enzymes in Metabolic Reactions
Phosphatase enzymes play a crucial role in various metabolic processes by releasing phosphate groups, increasing their availability for energy synthesis and cell structure formation. Acid phosphatases, with an optimum pH below 7.0, can be extracted from plant tissues like germinating mung beans. A protocol involving the use of sodium carbonate and a colorimeter for measuring absorbance in enzyme reactions is outlined. The process includes preparing enzyme extracts and substrates, conducting the enzyme reaction, and utilizing a colorimeter to measure absorbance at specific wavelengths.
Download Presentation
Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
Presentation Transcript
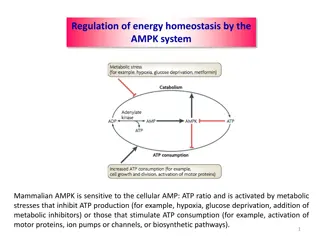
Background Phosphatase enzymes are involved in a range of metabolic reactions. A key function of these enzymes is to release phosphate groups into the metabolic pool thereby increasing their availability for use in a range of processes including ATP synthesis and membrane construction.
Background Phosphatase is one of the enzyme systems mentioned into the CfE Higher and Higher Human course specification.
Background Acid phosphatases (those with an optimum pH <7.0) can be extracted from a range of plant tissues germinating mung beans or bean sprouts are a cheap and reliable source. The substrate is phenolphthalein bisphosphate (PPP). Under suitable conditions, phosphatase catalyses the breakdown of PPP to form phenolphthalein (PP).
At neutral or acidic pH, the products of the above reaction (PP and phosphate) are both colourless so their presence is difficult to detect. This can be overcome through the addition of sodium carbonate which has 2 effects: 1) raising the pH of the solution >pH10 with cessation of enzyme activity 2) converts PP to its anionic form, which is pink.
Brief protocol outline 1. Add 1 cm3 sodium carbonate solution to 7 cuvettes. 2. Prepare enzyme extract and substrate 3. Mix enzyme extract with buffer 4. Add 1 cm3 enzyme/buffer mixture to cuvette 1 Blank 5. Add substrate solution to the mixture of enzyme/buffer. 6. At 2 minute intervals, remove 1cm3 of the substrate/enzyme/buffer mixture and add to one of the cuvettes. 7. Measure the absorbance at 550 nm.
Colorimeter Biochrome (550nm) To measure absorbance, ensure the colorimeter has Abs on the display for absorbance. If not, press the Abs / %T button (T transmission). The colorimeter has a range of LED light sources emitting light of specific wavelengths. Turn the wheel on the right hand side until 550nm is displayed above the cuvette holder. When reading the colorimetric blank, press and hold R (Reference) should produce a reading of 0.00 When reading a sample, press T (Test).
Part A Preparing the enzyme extract Materials 20g Bean sprouts Distilled water Pestle and mortar Scissors plastic pipette 6x microfuge tubes Microcentrifuge Marker pen Bijou labelled Enzyme extract
Germination if using mung beans (would use about 30 per extraction)
Take ~20g bean sprouts and place them into a mortar. Remove and discard the green seed case (testa) if it is still attached.
Add 5 cm3 distilled water and grind the bean sprouts with a pestle to achieve a smooth paste.
Cut the end of a 3 cm3 plastic pipette. Divide the bean sprout mixture equally between 6 microcentrifuge tubes. Should be approximately equal volume to ensure the centrifuge rotor is balanced. Close the lids and label with your initials.
Centrifuge your samples for 5 minutes to produce a pellet.
Carefully remove the supernatant from the microfuge tubes (using a clean disposable pipette) and pool their contents in a clean bijou marked enzyme extract .
To carry out the assay at pH5 and pH7, at least 5 cm3 enzyme extract is required. If the supernatant is less than this volume, add some distilled water. A bijou holds 7 cm3.
Part B the phosphatase assay Materials Enzyme extract (at least 5 cm3) 10 cm3 citric acid/phosphate buffer, pH 5.0 10 cm3 citric acid/phosphate buffer, pH 7.0 6 cm3 0.2% phenolphthalein phosphate 25 cm3 10% (w/v) sodium carbonate Stopclock Waterbath (30 C) Paper towels 14x absorption cuvettes Cuvette rack Colorimeter (we are using a Biochrome colorimeter 550 nm) Automatic pipette and clean tips Polystyrene cup (or similar)
Pipettes: The volumes outlined in the following steps should be dispensed using the automatic 1 cm3 pipette with a clean tip. Temperature: All solutions should be kept at 30 C throughout the assay.
Transfer water from the waterbath (30 C) to the polystyrene cup. Stand the universal of buffer (pH 5.0), bijou of enzyme extract and bijou of substrate (PPP) in the cup.
Using the 1 cm3 automatic pipette, add 1 cm3 sodium carbonate into 7 cuvettes. Discard the tip.
Transfer 2 cm3 enzyme extract to the buffer solution in the universal bottle.
For the colorimetric blank: transfer 1 cm3 of the mixture from the universal (buffer + enzyme) to the first cuvette.
Transfer 2 cm3 PPP to the enzyme/buffer mixture. Start the stopwatch. Mix the contents thoroughly but without creating too many bubbles.
At 2 minute intervals, transfer 1 cm3 of the PPP/enzyme/buffer mixture to a cuvette. The sodium carbonate will stop the reaction.
Use the colorimetric blank to zero the colorimeter (contains buffer and enzyme cuvette 1). Measure the absorbance of the remaining solutions at 550 nm.
Results Time (min) Time (min) Absorbance Absorbance pH 5.0 pH 7.0 0 2 4 6 8 10 12
Results our results Time (min) Time (min) Absorbance Absorbance pH 5.0 0.00 0.09 0.18 0.22 0.31 0.42 0.56 pH 7.0 0.00 0.02 0.04 0.07 0.12 0.19 0.26 0 2 4 6 8 10 12
Effect of pH on phosphatase activity in bean sprouts 0.6 0.5 0.4 Absorbance 0.3 pH 5.0 pH 7.0 0.2 0.1 0 0 2 4 6 8 10 12 Time (min)
Results Effect of pH on mung bean phosphatase activity 0.25 Relative Activity 0.2 0.15 0.1 0.05 0 2 3 4 5 6 7 8 9 pH
Other independent variables? oEffect of temperature oPhosphatase levels from different species oTest a range of pH values at a fixed time point (~10-12 minutes) oEffect of phosphate concentration to look at end-product inhibition.