HIV Opportunistic Infections and Prophylaxis Overview
Opportunistic infections (OI) are a major concern for individuals with HIV, often accelerating disease progression and causing severe complications. Prophylactic measures such as TB prophylaxis, Clo-trimazole, and Fluconazole are essential to prevent OIs and improve the overall health outcomes of people living with HIV.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
HIV OI's and prophylaxis, PEP, PREP COMPILED BY JASON VERMAAK, EKTHA GOVIND AND ANJANA THOMAS
Opportunistic infection In HIV Opportunistic infections are those that occur more frequently in immunocompromised populations and are often more clinically severe in this group of people. There is a correlation with CD4 count, hence prophylaxis is mainly offered to individuals with CD4 less than 200. Opportunistic infections is often considered as AIDS- defining illnesses.
Opportunistic infections Cryptococcal meningitis Toxoplasmosis PCP Oesophageal candidiasis Certain cancers, including Kaposi s sarcoma, Lymphomas and cervical Cryptosporidiosis Cystoisosporiasis Cytomegalovirus Herpes simplex virus Histoplasmosis Tuberculosis Mycobacterium avium complex Progressive multifocal leukoencephalopathy Toxoplasmosis And so on. . .
Why consider prophylaxis? Morbidity and mortality of these opportunistic infections are severe OI s accelerate the progression of disease by causing further damage to the body and the complications can be long term Ulcerative lesions can facilitate spread of HIV
TB prophylaxis All HIV patients irrespective of CD4 count Exclusions: Suspected or confirmed TB Peripheral neuropathy Liver disease Alcohol abuser Previous MDR or XDR TB INH 300mg dly for 12 months + pyridoxine 25mg dly Pregnant women CD4 more than 350, defer prophylaxis until after delivery CD4 less than 350, initiate prophylaxis
Clo-trimazole Prophylactic against many infections such as: PCP, Toxoplasmosis and cystoisosporiasis Indications: WHO clinical stage 2,3 or 4 CD4 less than 200 After 1 year, if CD4 more than 200 then stop this prophylaxis Clotrimazole 160/800mg po dly
Fluconazole Used for cryptococcal meningitis NHLS labs automatically do a CrAg test if CD4 is less than 100 If CrAg positive and no symptoms of meningitis: Treat with fluconazole 1200mg dly 14 days, then 800mg dly for 8 weeks, then 200mg dly for at least 1 year. After 1 year if CD4 is more than 200 treatment can be stopped. In resource limited setting (No CrAg test): Fluconazole prophylaxis 200mg po dly until CD4 more than 200.
Pre exposure prophylaxis (PrEP) PrEP is the use of anti retrovirals by HIV negative individuals before potential exposure to HIV to prevent them from acquiring HIV Criteria to initiate PrEP HIV negative Risk of HIV infection Willing and able to adhere to PrEP Prepared to repeat RVD testing every 3 months No contra-indications to tenofovir or emtricitabine
PrEP cont. . . Contra-indications HIV positive Creatinine clearance less than 60 Use of other nephrotoxic agents Young individuals less than 35kgs or less than 15 years of age who are less than Tanner stage 4 Tests required before initiation HIV test Creatinine Hep B surface antigen Pregnancy test
PrEP cont. . . Regimen: Tenofovir 300mg dly Emtricitabine 200mg dly
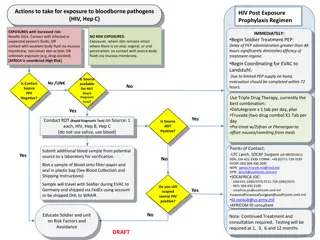
Post-Exposure Prophylaxis (PEP) Occupational exposure and HPCSA guidelines Increased risk of transmission in health care workers Transmission through infected blood or other bodily fluids Risk reduced through effective IPC measures HPCSA guidelines PEP must be available where increased risk for transmission is present All healthcare institutions must have clear and concise policy on PEP Students at risk should be insured either by university or training site Universal precautions should be followed and correct tools supplied by the institution
PEP cont. . . Assess risk Indicated Deep percutaneous sharp injuries Percutaneous exposure involving hollow needle used in vein or artery Visible blood on sharp instrument involved in percutaneous injury Source patient has terminal disease or with high viral load Contact of bodily fluid (exclude urine, saliva and sweat) and blood with non intact skin or mucous membranes (in particular conjunctiva) Not indicated The material exposed to the health care worker not infectious for HIV Patient not infected and no features of seroconversion Health care worker positive
PEP cont. . . Baseline test FBC U/E (creat) HEP studies: Heb B Surface AB, HCV AB Syphillis serology HIV rapid
PEP cont. . . Regimen (all for 4 weeks) Tenofovir 300mg dly (provided eGFR more than 50) Lamividine 200mg dly Dolutegravir 50mg dly In women of childbearing age, pregnant women (less than 6 weeks) or if dolutegravir not tolerated: Tenofovir 300mg dly Emitricitabine 200mg dly Atazanivir/Ritonavir 300/100mg dly OR Lopinavir/ritonavir 200/50mg 2 tabs bd If Tenofovir contraindicated or patient failing Tenofovir regimen replace Tenofovir and emtricitabine with zidovudine 300mg bd and lamivudine 150mg bd If source patient is failing ART regimen adjust PEP
PEP cont. . . Follow-up 2 weeks If tenofovir is part of PEP then check creatinine If AZT is part of PEP then check FBC 6 weeks HCV PCR HIV ELISA 4 months HIV ELISA
References Standard Treatment Guidelines and Essential Medicines List for South Africa, Hospital Level, Adults 2019 Edition file:///C:/Users/ThinkPad%20X280/Desktop/PrEP%20Guidelines%20Fin al%2020%20Aug%202019.pdf https://www.hpcsa.co.za/Uploads/Professional_Practice/Ethics_Boo klet.pdf https://www.hpcsa-blogs.co.za/wp-content/uploads/2017/04/2017- IN-Handbook-ANNEXURE-H.pdf