Understanding Scientific Method and Units in Chemistry.
Learn about designing controlled experiments, identifying variables, and understanding constants through scenarios involving bacteria cultures and pizza grease comparison. Explore the differences between theory and law in scientific context, and discover the base units and metric units used in chemistry for measurement. Understand the units for volume and how to calculate it for different types of substances.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
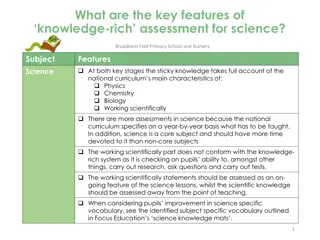
MATTER AND MEASUREMENT
How is a controlled experiment designed? A controlled experiment should have a control, a standard for comparison. Controlled experiments only change one variable at a time. The one variable that changes in a controlled experiment is called an manipulated variable. The thing that is affected by changing the manipulated variable is called the responding variable. All of the things that stay the same in an experiment are called constants.
Read the scenario below: Students put the same type of bacteria in 15 petri dishes. Antibiotic A was applied to 5 dishes, Antibiotic B was applied to 5 dishes, and antibiotic C was applied to the last 5 dishes. At the end of two weeks, the number of bacteria cultures were counted.
1.What were the constants? 2.What was the manipulated variable? 3.What was the responding variable? 4.Was there a control?
Which pizza has the most grease? Lucy and Amy buy three slices of cheese pizza: one from Dominoes, one from Pizza Hut and one from Jules. The hold each slice over a paper towel for 60 seconds and let the grease drip down onto a napkin. The observe to see which napkin has the most grease. Then they eat the pizza!
How does theory differ from a law? Theory Law
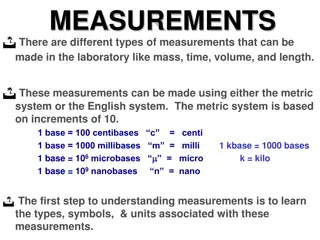
What are the base units used in chemistry? Meter is used to measure length Kilogram is used to measure mass Kelvin is used to measure temperature Second is used to measure time
What are the metric units of length? Kilo Hecto Deka Meter Liter Gram 1 Over Deci Centi Milli 1000 Kids 100 Have 10 Dropped .1 Dead .01 Converting .001 Metrics
What are the units for volume? 1 Liter (L) = 1000mL 1000 mL = 1 Liter (L) 1 cm3= 1 mL Volume of solids = Length X Width X Height Volume of liquids = Use a graduated cylinder Volume of gas /Volume of irregular solids = Use displacement of water
What are the units for mass? 1 Gram (g) = 1000 mg 1 kg = 1000 grams Mass of solids = use electronic scale Mass of Liquids= measure container first, then zero and add liquid
What are the units for temperature? C = K -273 K = C + 273 F =9/5 C + 32 C=5/9 (F - 32)
What is a conversion factor? A ratio of equivalent measurements used to solve problems 1 meter = 100 cm 1 meter = 1 meter
What is dimensional analysis? A way to analyze and solve problems using the units or dimensions of the measurements. You need conversion factors!
Sample Problems: How many seconds in a 24 hour day:
What are the two kinds of observations? Qualitative Observations describe an object s characteristics, properties or attributes. Examples would include: color, texture or odor. Quantitative Observations involve a quantity or an amount. Examples would include mass , volume or temperature.
Why are measurements expressed in scientific notation? Scientific notation makes large numbers easier to work with.
Sample Problems: 345.89 = .00034 = 23.004 =
How do you evaluate accuracy and precision? Accuracy Compare the measured value to the correct value Precision Compare the values of two or more repeated measurements Arrows hit the bullseye Arrows all hit the same point
How is percent error calculated? Experimental Value - Accepted Value Accepted Value X 100%
How is density determined? Units g/mL or g/cm3
Sample Problems: The mass of a marble is 5.6 grams. The volume is determined using displacement of water and is found to be 4.8 mL. What is the density of the marble?
What are some examples of physical properties? Color Melting Point Boiling Point
What are the three states of matter and how do they compare Solid Liquid Gas Has a definite shape and volume Particles are tightly packed incompressible Takes the shape of container Particles are close together but not rigid or orderly Has a definite volume Not easily compressed Fills the container Takes the shape of container Particles are far apart Easily compressed
What are some examples of physical and chemical changes? Physical Change Tearing Folding Evaporation/Freezing Dissolving Chemical Change Flammability Rusting
What are some signs that a chemical change has occurred? Color Change Precipitate (solid) Energy Transfer (heat) Bubbles
What are the two kinds of mixtures? Homogeneous Appears the same throughout Includes solutions Can be liquids, solids and gases Heterogeneous Does not appear the same throughout
How can mixtures be separated? Filtering-separates solids from liquids Density Method- separates solids from solids or liquids Distillation- separates liquids from liquids
How do elements, compounds and mixtures compare? Elements- one kind of atom Compounds- one or more atoms Mixtures- two or more substances