Understanding Scientific Notation in Mathematics and Chemistry
Scientific notation is an essential concept for representing very large or very small numbers efficiently. It simplifies numbers by expressing them as a coefficient multiplied by a power of 10. This summary covers the basics of scientific notation, from converting numbers to and from scientific notation to understanding significant figures in mathematical and chemical contexts.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Math and Chemistry Yay for math!
How wide is our universe? 210,000,000,000,000,000,000,000 miles (22 zeros) This number is written in decimal notation.
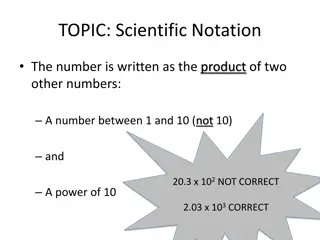
What is scientific notation? An easier way to write larger numbers Scientific notation form a x 10n a is between 1 and 10 n is an integer
How to write scientific notation Width of universe is 210,000,000,000,000,000,000,000 miles Where is the decimal place? After the last zero Where does it need to go so the number is between 1 and 10? - Between the 2 and the 1
How do we turn that number into a number between 1 and 10? Move the decimal place 23 times forward 2.10,000,000,000,000,000,000,000.
How do we write this number in scintific notation? When the original number is MORE than 1, the exponent is positive. 210,000,000,000,000,000,000,000 The answer in scientific notation is 2.1 x 1023
What if the original number is less than one? 0.0000000902 Where should the decimal go to make the number between 1 and 10? 9.02 How many times was the decimal moved? 8 When the original number is less than 1 the exponent is negative 9.02 x 10-8
Examples 28750.9 0.00567 243.56
How do you go back to decimal notation? 1.8 x 10-4 move your decimal 4 places forward 0.00018
Examples 4.58 x 106 2.3487 x 102 8.65 x 10-4
What are significant figures? How many numbers in a given amount are significant
What are the rules? #1 : If there are NO decimal point start at the RIGHT and count, beginning with the first non- zero digit. 340 30400 34955 #2: If there IS a decimal point start at the LEFT and count, beginning with the first non-zero digit. 340. 30400. 0.34955 5 0.00040 2 2 3 5 3 5
How many sig figs? 7 40 0.5 0.00003 7 x 105 7,000,000 1 1 1 1 1 1
How many sig figs here? 1.2 2100 56.76 4.00 0.0792 7,083,000,000 2 2 4 3 3 4
How many sig figs here? 4 2 5 3 3 6 3401 2100 2100.0 5.00 0.00412 8,000,050,000
How to add and subtract? 1) Count the number of significant figures in the decimal portion of each 2) Add or subtract the numbers 3) Round the answer to the LEAST number of places in the decimal portion of any number in the problem.
Add/Subtract examples 2.45cm + 1.2cm = 3.65cm 3.7 cm 7.432cm + 2cm = 9.432 cm 9 cm
How to Multiply and Divide? The LEAST number of significant figures in any number of the problem determines the number of significant figures in the answer
Examples 56.78 cm x 2.45cm = 139.111 cm2 4 3 139 cm2 75.8cm x 9.6cm = 727.68 cm2 3 2 730 cm2
What are metrics? A conversion method to convert between units
What are the basic units of measurements? Distance is measured in meters (m) Volume is measured in liters (l) Weight is measured in grams (g) Time is measured in seconds (s)
What are the prefixes for measurements? Kilo Hecto Deka Base deci centi milli K H D d c m
What are the Abbreviations for measurements? meter = m liter = l gram= g Km = kilometer cl = centiliter Dg = dekagram dm = decimeter g = gram Hl = mm = l = cg = Kg =
How to remember the order of prefixes? Kilo Hecto Deka base deci centi milli Gram Liter meter King Henry Died by drinking chocolate milk
Ladder Method 1 2 3 KILO 1000 Units HECTO 100 Units DEKA 10 Units DECI 0.1 Unit CENTI 0.01 Unit MILLI 0.001 Unit Meters Liters Grams 4 km = _________ m How do you use the ladder method? 1st Determine your starting point. Starting Point Ending Point 2nd Count the jumps to your ending point. How many jumps does it take? 3rd Move the decimal the same number of jumps in the same direction. __. __. __. = 4000 m 4. 2 1 3
Conversion Practice Try these conversions using the ladder method. 1000 mg = _______ g 1 L = _______ mL 160 cm = _______ mm 14 km = _______ m 109 g = _______ kg 250 m = _______ km Compare using <, >, or =. 56 cm 6 m 7 g 698 mg
Metric Conversion Challenge Write the correct abbreviation for each metric unit. 1) Kilogram _____ 4) Milliliter _____ 7) Kilometer _____ 2) Meter _____ 5) Millimeter _____ 8) Centimeter _____ 3) Gram _____ 6) Liter _____ 9) Milligram _____ Try these conversions, using the ladder method. 10) 2000 mg = _______ g 15) 5 L = _______ mL 20) 16 cm = _______ mm 11) 104 km = _______ m 16) 198 g = _______ kg 21) 2500 m = _______ km 12) 480 cm = _____ m 17) 75 mL = _____ L 22) 65 g = _____ mg 13) 5.6 kg = _____ g 18) 50 cm = _____ m 23) 6.3 cm = _____ mm 14) 8 mm = _____ cm 19) 5.6 m = _____ cm 24) 120 mg = _____ g
What is Accuracy? Accuracy refers to how closely a measurement matches the true or actual values To be accurate only requires the true value (bulls eye) & one measurement (for the arrow to hit the target)
What is Precision? Precision is when you have multiple measurements that are close together.
Accuracy & Precision In order to be accurate and precise, one must pay close attention to detail to receive the same results every time as well as hit the target .
Comparing Accuracy & Precision Notice the difference in these pictures. To win the tournament the archers must hit the target the most times. The winner must show accuracy & precision. The 1st archer has _____ accuracy & ____ precision. The 2nd archer has _____ accuracy & ____ precision. The 3rd archer has _____ accuracy & ____ precision. The 4th archer has _____ accuracy & _____ precision BAD BAD BAD GOOD GOOD GOOD GOOD BAD
Example 1 A sample is known to weigh 3.182 g. Jane weighed the sample five different times with the resulting data. Which measurement was the most accurate? 3.200 g 3.180 g 3.152 g 3.189 g
What is temperature? The measure of the average kinetic energy of any particle
What are the units of temperature? Fahrenheit (oF) Celsius (oC ) Kelvin (K)
How to convert between Celsius and kelvin Formula: K= oC + 273
Example Convert 0oC into K K = oC + 273 K = 0oC + 273 K = 273 273 K
Example 2 Convert 35oC into K K = oC + 273 K = 35oC + 273 K = 308 308K
Example 3 Convert 467K into oC K = oC +273 oC = K 273 oC = 467K 273 oC = 194 194oC
What is dimensional analysis? A way to convert between measurements and units
How many minutes are in 3.61 hours? 1. Write the given. 2. Draw the chart.
3. Think of a relationship: 1 hr = 60 min 4. Cancel units diagonally.
How to do Multi- step problems Calculate the number of seconds in two weeks
Your turn 1849 yards = ________________ miles Hint 5280 ft = 1 mile 3 feet = I yard
What is density? Density is defined as mass per unit volume. It is a measure of how tightly packed and how heavy the molecules are in an object.
How to find density? Density = mass Volume Units of density are g / cm3 or g/ml
Example 1 If the mass of an object is 35 grams and it takes up 7 cm3 of space, calculate the density