Unraveling the Anomeric Effect: Origins and Mechanisms
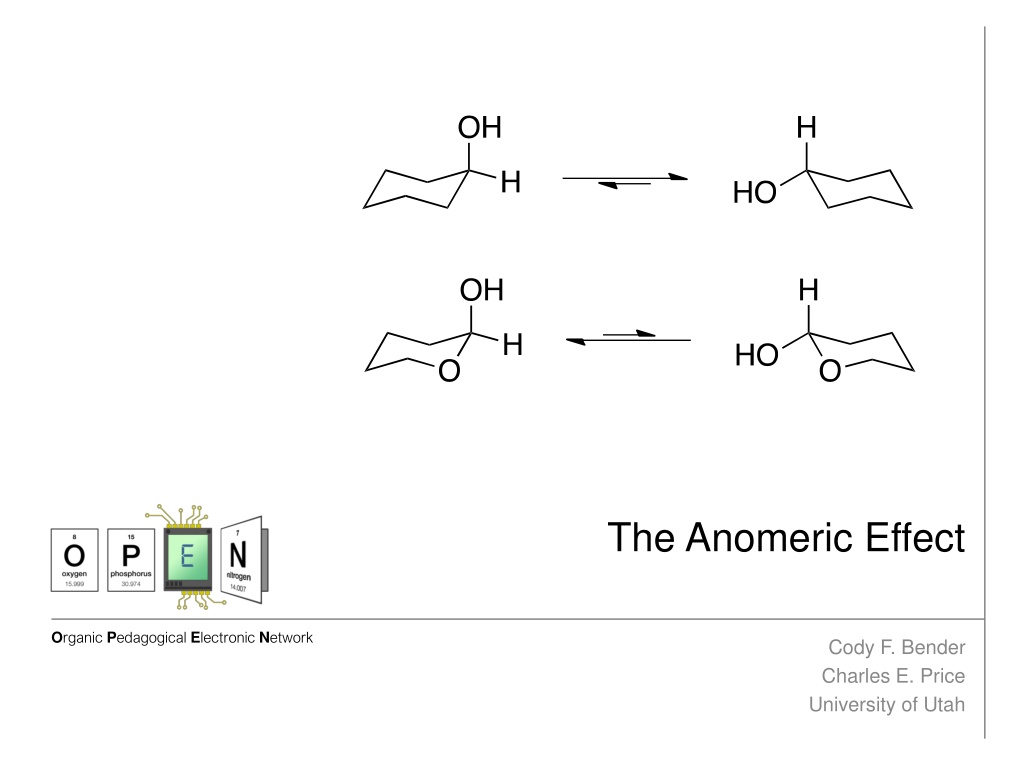
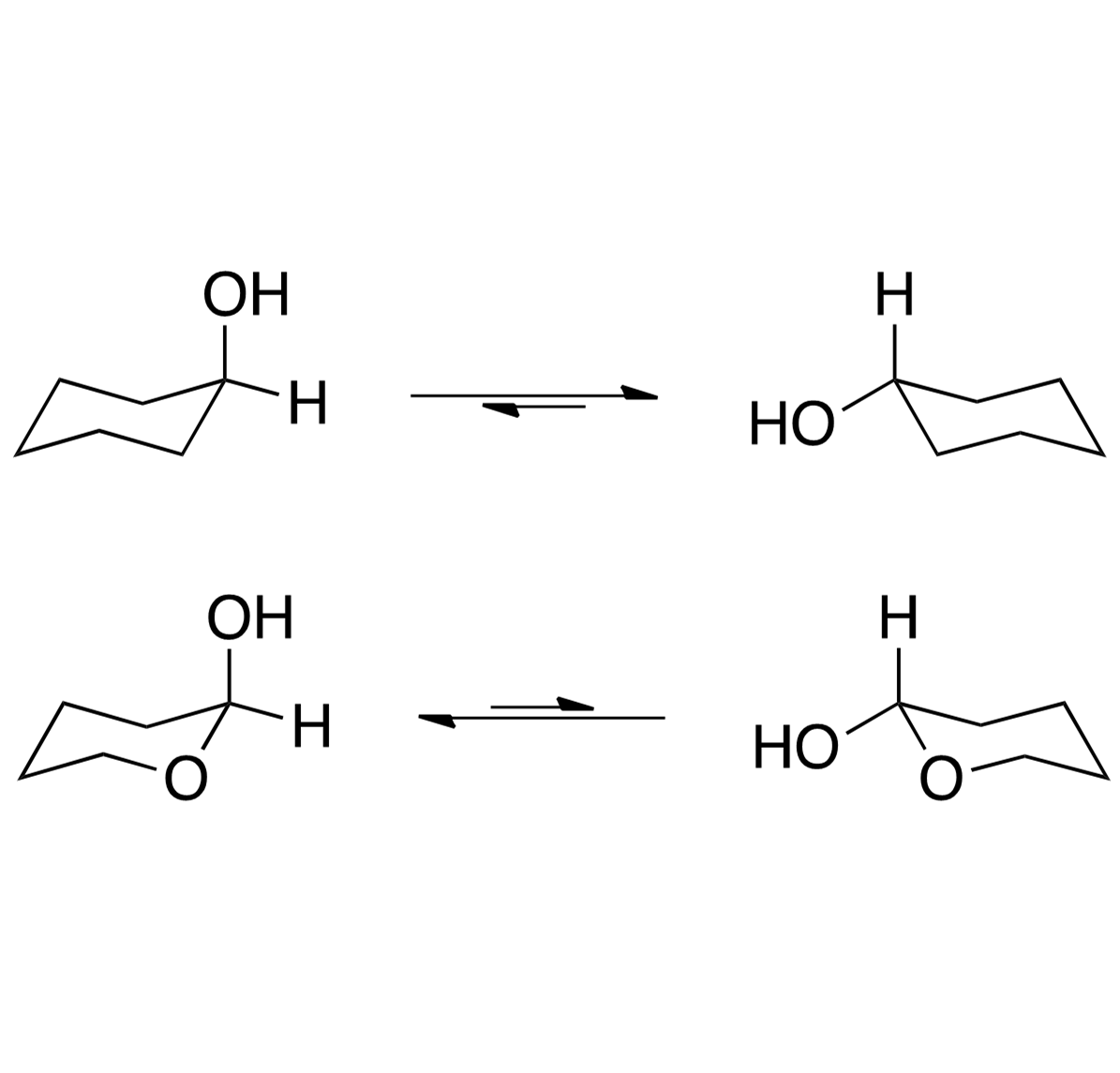
The anomeric effect, discovered in 1955, reveals the preference of certain substituents in cyclohexyl systems to occupy the axial position. This phenomenon was first observed by J.T. Edward, N.-J. Chu, and R.U. Lemieux, challenging existing conceptions. The effect is attributed to hyperconjugation and dipole minimization, influencing the conformation of sugar molecules. Electrostatic interactions between lone pairs and adjacent carbons play a crucial role, leading to a reduced dipole moment. These findings have advanced our understanding of molecular conformations and chemical reactivity.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
The Anomeric Effect Organic Pedagogical Electronic Network Cody F. Bender Charles E. Price University of Utah
Discovery of the Anomeric Effect The anomeric effect was discovered in 1955 with the work of J.T. Edward, N.-J. Chu, and R.U. Lemieux. Edward notice alkoxypyranose rings favored axial positioning, which contradicted previous notions1 He was the first to state lone pairs affected conformation by suggesting the cyclic oxygen s lone pairs interacted with substituents1 Edward s observed axial preference 1Edward, J.T. Chem. Ind. (London) 1955, 1102. 2Lemieux, R.U. Explorations with Sugars-How Sweet It Was, In Profiles, Pathways, and Dreams, Seeman, J.I., Ed.; American Chemical Society: Washington D.C., 1990. 3Lemieux, R.U.; Chu, P. Abstracts of Papers; 133rd National Meeting of the American Chemical Society, San Francisco, CA.; American Chemical Society: Washington D.C., 1958; 31N.
Discovery of the Anomeric Effect Chu and Lemieux noticed a similar effect while observing equilibrium of acetylated aldohexopyranose rings2 The measured energy difference of - and -pyranose rings to be 0.94 kcal/mol, favoring the - conformer (Figure 3)2 It was also measured that the free energy difference was 1.48 kcal/mol for -xylose2. Chu and Lemieux s acetylated aldohexopyranose results The term anomeric effect was first coined at an ACS meeting in 1958 due to the frequent occurrence of this phenomenon on the anomeric carbon of sugar molecules3 1Edward, J.T. Chem. Ind. (London) 1955, 1102. 2Lemieux, R.U. Explorations with Sugars-How Sweet It Was, In Profiles, Pathways, and Dreams, Seeman, J.I., Ed.; American Chemical Society: Washington D.C., 1990. 3Lemieux, R.U.; Chu, P. Abstracts of Papers; 133rd National Meeting of the American Chemical Society, San Francisco, CA.; American Chemical Society: Washington D.C., 1958; 31N.
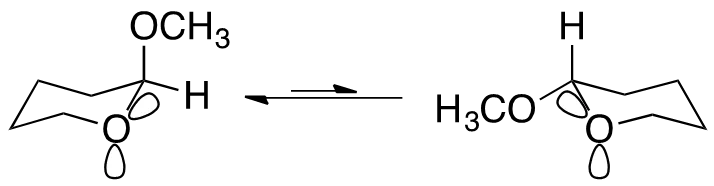
The Anomeric Effect Defined The anomeric effect describes the tendency for electronegative substituents geminally bound to other heteroatoms within a cyclohexyl system to prefer the axial position. Hyperconjugation Hyperconjugation is believed to be the prominent factor for the anomeric effect4 Shown is where the cyclic heteroatom s lone pairs donate electron density to the * oribtal of the adjacent carbon-substituent bond, known as a *- interaction4 Due to orbital alignment, this interaction is not possible with equatorial subsitution4 - * interaction 4Cuevas, E.; Juaristi, G. (1995). The Anomeric Effect. Boca Raton: CRC Press.
The Anomeric Effect Defined The anomeric effect describes the tendency for electronegative substituents geminally bound to other heteroatoms within a cyclohexyl system to prefer the axial position. Dipole Minimization Another contributing argument is the result of a reduced dipole of the molecule due to lone pair orientation4 (Figure 5) Dipole minimization 4Cuevas, E.; Juaristi, G. (1995). The Anomeric Effect. Boca Raton: CRC Press.
Overcoming the Anomeric Effect Since the anomeric effect only gives a benefit of 1-2 kcal/mol, it can easily be overcome by solvent and in synthesis. Solvent % Axial Solvent Effects CCl4 2.2 83 C6H6 2.3 82 Since equatorial conformers give larger dipoles, solvents with larger dielectric constants, or polarity, can aid in overcoming the anomeric effect5 CS2 2.6 80 CHCl3 4.7 71 CH3COCH3 20.7 72 The table shows this trend using 2- methoxytetrahydropyran in various solvents CH3OH 32.6 69 CH3CN 37.5 68 H2O 78.5 52 Solvent effects on axial preference 5Lemieux, R.U.; Pavia, A.A.; Martin, J.C.; Watanabe, K.A. Can. J. Chem. 1969, 47,4427. 6Koenigs, W.; Knorr, E. Eur. J. Inorg. Chem. 1901, 34, 957.
Overcoming the Anomeric Effect Since the anomeric effect only gives a benefit of 1-2 kcal/mol, it can easily be overcome by solvent and in synthesis. Anti-Anomeric Synthesis Various reactions have been developed to produce products that evade the anomeric effect due to low relative energetic benefits, coming from high energy contributions of sterics in the case of a ring flip.6 Koenigs-Knorr Reaction5 5Lemieux, R.U.; Pavia, A.A.; Martin, J.C.; Watanabe, K.A. Can. J. Chem. 1969, 47,4427. 6Koenigs, W.; Knorr, E. Eur. J. Inorg. Chem. 1901, 34, 957.
Example of the Anomeric Effect in Avermectin 1b The avermectins are a class of 16-membered macrocyclic lactones with anthelmintic and insecticidal properties. They are natural products that can be isolated from the bacteria Streptomyces avermitilis.7,8William C. Campbell and Satoshi Omura were awarded the Nobel Prize for Medicine in 2015 for these molecules discovery. The avermectins show three different examples of the anomeric effect, highlighted in red. Two occur in the disaccharide moiety and the third occurs in the spiroketal structure, in which the anomeric effect is not entirely observed. The external ring of the spiroketal follows the anomeric effect as a substituent. But, the isopropyl group on this ring disrupts the ability for the internal ring to occupy the axial position.9 Structure of avermectin 1b3 7Omura, S.; Shiomi, K. Pure and App. Chem. 2007. 79. 581. 8Pitterna, T. et al. Bioorg. Med. Chem. 2009. 17. 4085. 9Springer, J.; Arison, B.; Hirshfield, J.; Hoogsteen, K. J. Am. Chem. Soc. 1981. 103. 4221.
Practice Problems 1) Predict the Keq for the following equilibrium. a) Keq > 1 b) Keq = 1 c) Keq < 1 2) What is the preferred position of the allyl substituted anomeric carbon on this tri-methoxybenzylpyranose ring? a) Axial b) Equatorial c) No preference 10Shuto, S. Angew. Chem. Int. Ed. 2003. 42. 1021-1023. 3) What is the position of the vinyl group in the product of the given reaction? a) Racemic b) Axial c) Equatorial 11Aponick, A. Chem. Eur. J. 2013. 19. 11613-11621. Solutions: 1) C, 2) A, 3) B
Contributed by: Cody F. Bender, Charles E. Price (Undergraduates) University of Utah, 2016 This work is licensed under a Creative Commons Attribution- ShareAlike 4.0 International License.