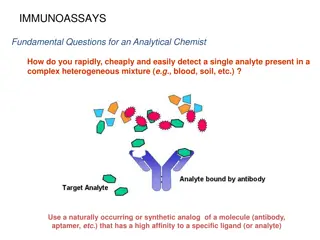
Understanding Analytical Chemistry: Solutions and Miscibility
Explore the fundamentals of solutions in analytical chemistry, including the types of solutions, role of solutes and solvents, and the concept of miscibility. Learn about the six types of solutions based on phases, the general rule of miscibility, and the solution process involving dissolution and crystallization equilibrium. Dive into Le Chatelier's principle and its application in equilibrium systems.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Analytical Chemistry Analytical Chemistry By Dr. Jamal Ahmed Abdel Barry Professor in Clinical Biochemistry
Advanced Analytical Chemistry Solutions, Molarity, Percent Solutions, Molality And Normality
Solution : A homogeneous mixture of 2 or more substances. Examples: Salt water, Orange juice. All solutions are a mixture of the solute(s) and the solvent. They are always listed with the lesser constituent (the solute) first.
Solute: The substance that is dissolved. It is the lesser constituent by volume. (Ex: The Salt in the Saltwater.) Solvent : The substance that does the dissolving. It is the greater constituent by volume. (Ex: The Water in the Saltwater.)
There are 6-types of solutions based on the phases of their components. 1. Gas - Gas 2. Liquid - Gas 3. Gas - Liquid 4. Liquid - Liquid 5. Solid - Liquid O2 in air Water vapor in the air CO2 in H2O 2-stroke fuel (oil in gas) Salt in water 6. Solid - Solid Alloys (Cu in Ag, Sterling Ag )
Miscible : Describes two liquids that are mutually soluble in each other. Examples: 1. Water and alcohol are completely miscible with each other. 2. Water and ether are slightly miscible with other. About 1.5% water : 98.5% Diethyl ether (m/m) each 3. Water and oil are not miscible with each other.
The General Rule with regards to Miscibility: Likes dissolve likes. 1. Polar solvents dissolve polar compounds. 2. Non-polar solvents dissolve non-polar compounds.
The Solution process involves two actions: 1. Dissolving 2. Crystallizing Equilibrium (as its relates to solvation)- The physical state where the two opposing processes of dissolving and crystallizing occur at equal rates.
Le Chteliers principle - When stress is applied to a system at equilibrium, the system shifts to relieve the stress. Example: As you add salt to water, it dissolves. However, as the concentration of salt approaches saturation, the rate of solvation slows.
Saturated solution - A solution in which the dissolved and un-dissolved substances are at equilibrium. (The solvent can no longer dissolve any additional solute. It will simply fall to the bottom of the solution.) The solubility of a substance determines when a solution will become saturated.
Solubility : The maximum amount of a solute that can dissolve in a given amount of solvent under specified conditions. Examples at 20 C : 1. 222 g of Silver nitrate / 100 g of water 2. 3.89 g of Barium hydroxide / 100 g of water 3. 0.1 69 g of CO2 / 100 g of water (at SP) 4. 0.0043 g of O2 / 100 g of water (at SP)
Factors that affect the rate of solution: 1. Particle size 2. Agitation 3. Temperature (aka: the Heat of Solution) 4. Polarities of solute and solvent 5. Concentration of solution (solvation slows as it saturation) nears
Heat of Solution: The difference between the heat content of a solution and it s components. Heats of solution are either positive or negative.
Positive Heat of Solution : Solute + solvent + heat solution (endothermic) Heating helps solvation Heat has a positive effect on solvation.
Negative Heat of Solution: Solute + solvent solution + heat (exothermic) Heating hinders solvation Heat has a negative effect on solvation.
Concentration units for solutions: The number of moles of solute per liter of solution 1. Molarity - 2. Molality - 3. Normality - The number of moles of solute per kilogram of solvent. The number of equivalents of a substance dissolved in a liter of solution. 4. Percent solutions - (m/m), (v/v), (m/v)