Techniques for Separating Mixtures: A GCSE Guide
Explore various separating techniques such as chromatography, distillation, filtration, and crystallization for different mixtures in a GCSE science context. Learn how to separate substances like solutes, solvents, liquids with similar boiling points, magnesium, salt, and more. Engage in a challenging task to separate copper from a mixture containing magnesium, salt, water, and copper using common laboratory equipment and chemicals.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
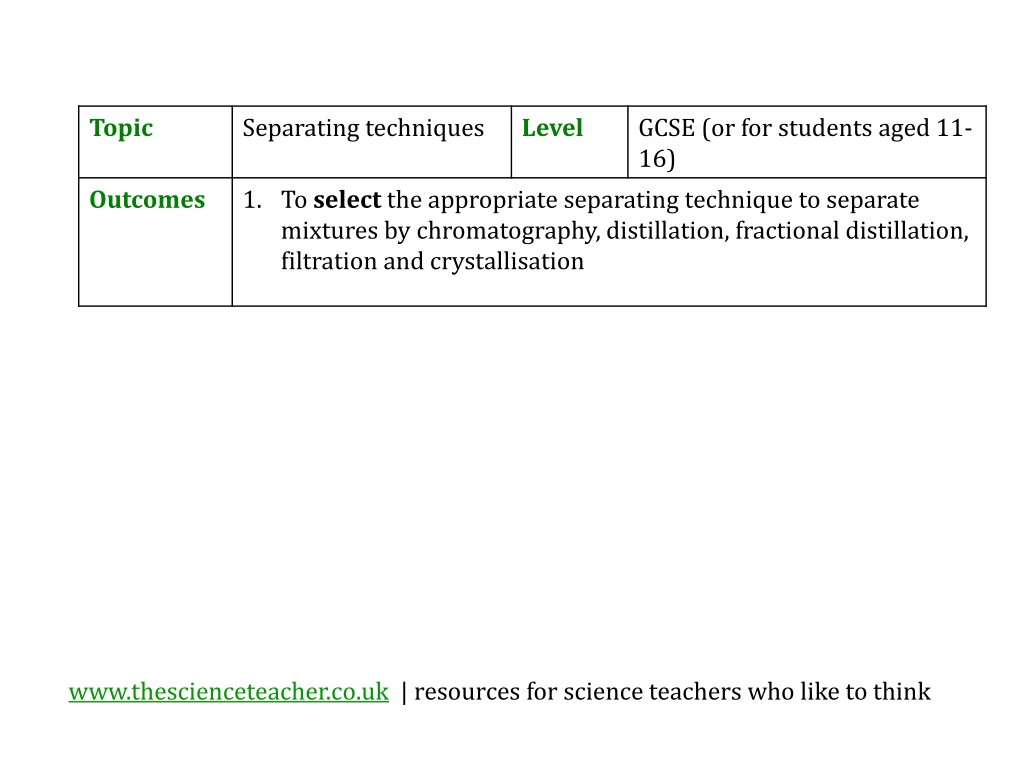
Topic Separating techniques Level GCSE (or for students aged 11- 16) Outcomes 1. To select the appropriate separating technique to separate mixtures by chromatography, distillation, fractional distillation, filtration and crystallisation www.thescienceteacher.co.uk | resources for science teachers who like to think
What to separate? Best method(s) of separation to use Solute from a solution Solvent from a solution Liquid A from a mixture containing three liquids A, B and C with similar boiling points Magnesium from a mixture of magnesium and salt Salt from a mixture containing salt, sand and water Pure water from a mixture containing salt, sand and water Copper (II) sulphate crystals from a mixture containing copper (II) sulphate and water Different pigments from plant leaves Iron filings from a mixture of sugar, iron and zinc
What to separate? Best method(s) of separation to use Solute from a solution Evaporation Solvent from a solution Distillation Liquid A from a mixture containing three liquids A, B and C with similar boiling points Magnesium from a mixture of magnesium and salt Salt from a mixture containing salt, sand and water Pure water from a mixture containing salt, sand and water Copper (II) sulphate crystals from a mixture containing copper (II) sulphate and water Different pigments from plant leaves Fractional distillation Dissolve in water and filter Filtration then Evaporation Distillation Crystallisation Chromatography Iron filings from a mixture of sugar, iron and zinc Use a magnet
Challenge! How would you separate copper from a mixture containing magnesium, salt, water and copper? You can use any chemicals and equipment found in the lab.
Challenge! 1. Filter to remove water and salt 2. Add dilute acid to react with magnesium 3. Filter again 4. Pure copper will be left