Understanding Polarity, Electronegativity, and Chemical Bonds
Delve into the concepts of polarity, electronegativity, and different types of chemical bonds by exploring the tug-of-war analogy and examples of polar, nonpolar, ionic, and covalent bonds. Learn how electronegativity values determine the nature of bonds and the sharing of electrons in molecules.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Tug of War Polarity and Electronegativity
Think about it Think about it What does the word POLAR mean to you?
Polar Bear Polar Bear North/South Poles North/South Poles
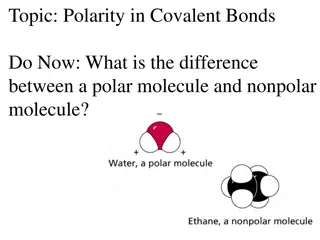
Polar Molecules Polar Molecules
What is What is Electronegativity? Electronegativity? Electronegativity: How much an atom in a molecule attracts electrons
Electronegativity Increases Electronegativity Decreases
Polarity Polarity Ionic bond: A bond in which the bonding electrons in the molecular orbital are transferred.
Polarity Polarity Covalent bond: A bond in which the bonding electrons in the molecular orbital are shared.
Polarity Polarity Nonpolar covalent bond: A covalent bond in which the bonding electrons in the molecular orbital are SHARED EQUALLY
Polarity Polarity Polar covalent bond: A covalent bond in which the bonding electrons in the molecular orbital are NOT SHARED EQUALLY
Electronegativity Electronegativity If the difference is less than 0.4, = nonpolar covalent. between 0.4 and 1.7 = polar covalent greater than 1.7 = ionic
Tug of War Tug of War Atom 1 EN value # people Atom 2 EN value # people Hypothesis Observation F Na F H P O As Cl F F
Atom 1 Atom 2 Electronegativity Difference Polar Nonpolar Ionic? Polar Covalent C 2.6 O 3.4 3.4 2.6 = .8
Agenda Agenda Unit 6 Topic 6 (Polarity) notes Forensics Lab! Who kidnapped Ms. O ??
February 15, 2008 February 15, 2008 What are the separation techniques that you know about?
February 19, 2008 February 19, 2008 Which of the following sets will dissolve in each other? Polar / Ionic Non Polar / Ionic Polar / Non Polar Non Polar / Non Polar Homework: Read Electronegativity Pgs 168-169 Take NOTES on what you read.
February 19, 2008 February 19, 2008 Which of the following sets will dissolve in each other? Polar / Ionic Non Polar / Ionic Polar / Non Polar Non Polar / Non Polar