Understanding Science, Matter, Energy, and Systems
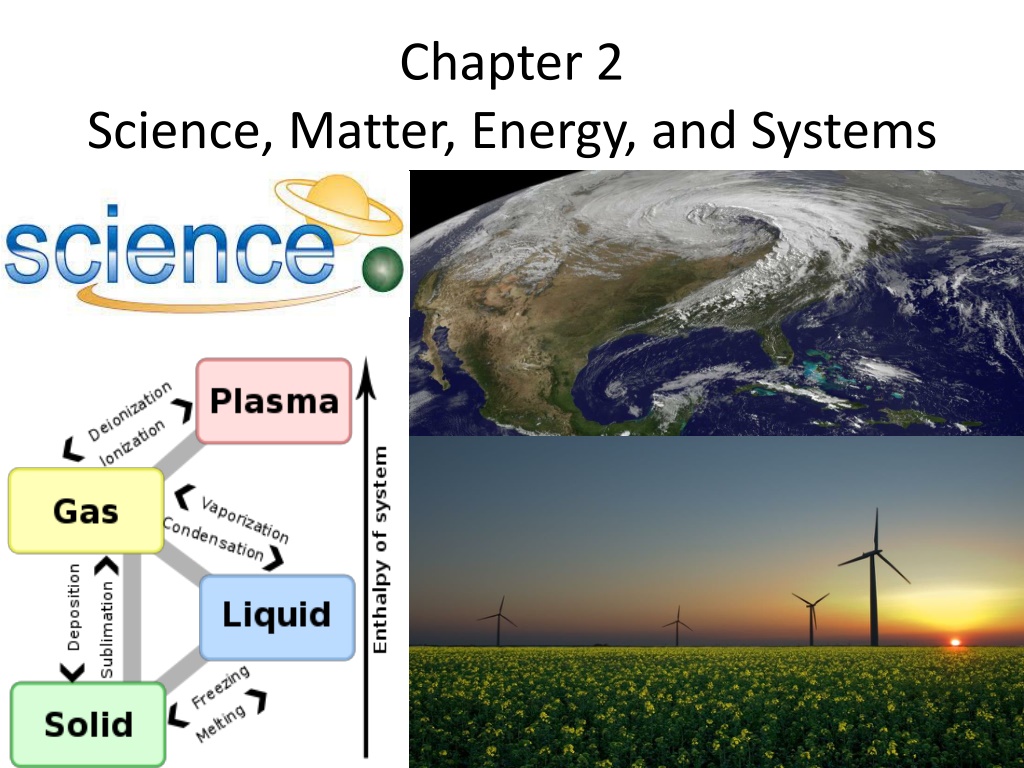
Science is an ongoing quest to understand the workings of nature through hypotheses, data, models, theories, laws, and peer review. Paradigm shifts occur when new scientific ideas replace old ones. Energy from the sun fuels Earth with solar energy entering ecosystems through photosynthesis. Elements and ions crucial to environmental science include hydrogen, carbon, oxygen, nitrogen, phosphorus, sulfur, and more.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Chapter 2 Science, Matter, Energy, and Systems
Science is an endeavor to discover how nature works. It has limitations and hypothesis are tentative and testable statements that must be capable of being supported or not supported by observational evidence. Data information required to answer the scientists questions Model an approximate representation or simulation of a system being studied. Scientific Theory a well-tested and widely accepted scientific hypothesis or a group of related hypothesis
Scientific Law a well tested and widely accepted description of what we find happening over and over again, the exact same way in nature. Peer Review scientists sharing information with other scientists working in the same field. Inductive Reasoning using specific observations and measurements to arrive at a general conclusion or hypothesis. Deductive Reasoning using logic to arrive at a specific conclusion based on generalization or premise.
Paradigm Shift when the majority of scientists in a field accept new ideas and discoveries and build a new framework for laws and theories, overthrowing older laws and theories. Tentative Science/Frontier Science hypotheses that have not been widely tested or studied and have not been accepted by peer review; tend to capture news and headlines. Reliable Science consists of data, hypotheses, theories, and laws that are widely accepted Unreliable Science -
Essentially 100% of the energy that fuels the earth comes from the sun EARTH S ENERGY BALANCE
Solar Energy Enters Ecosystems via Photosynthesis Terrestrial Ecosystems Aquatic Ecosystems PHYTOPLANKTON
Elements important to the Study of Environmental Science Hydrogen Carbon Oxygen Nitrogen Phosphorus Sulfur Chlorine Flourine Bromine Sodium Calcium Lead Mercury Arsenic Uranium
Ions Important to the Study of Environmental Science Hydrogen - H+ Sodium Na+ Calcium Ca2+ Aluminum Al3+ Ammonium NH4+ Chlorine Cl- Hydroxide OH- Nitrate NO3- Sulfate SO42- Phosphate PO43-
Compounds Important to the Study of Environmental Science Sodium Chloride NaCl Carbon Monoxide CO Carbon Dioxide CO2 Nitric Oxide NO Nitrogen Dioxide NO2 Nitrous Oxide N2O Nitric Acid HNO3
Compounds Important to the Study of Environmental Science Cont. Methane CH4 Glucose C6H12O6 Water H2O Hydrogen Sulfide H2S Sulfur Dioxide SO2 Sulfuric Acid H2SO4 Ammonia NH3
Law of Conservation of Matter When a chemical or physical change occurs, no atoms are created or destroyed. DUH.
Law of conservation of Energy (also known as the 1st law of thermodynamics) When energy is converted from one form to another in a physical or chemical change, no energy is created or destroyed. Once again, DUH. Energy Input always = Energy Output
2nd Law of Thermodynamics When energy changes from one form to another, we always end up with lower-quality or less usable energy than we started with. Energy always goes from a more useful to a less useful form when it is changed from one form to another.
What are Systems and how do they respond to change? System a set of components that function and interact in some regular way. Most systems have the following key components: Inputs from the environment Flows or throughputs of matter and energy within the system at certain rates Outputs to the environment
Feedback Loops Feedback any process that increases or decreases a change to a system. Feedback Loop when an output of matter, energy, or information is feed back into the system as an input and leads to changes in that system. Positive Feedback Loop system changes further in the same direction. (Decreasing Vegetaion in a valley) Negative Feedback Loop system changes in the opposite direction. (Your thermostat at home)
Negative/Positive Feedbacks http://www.bozemanscience.com/positive- and-negative-feedback-loops/