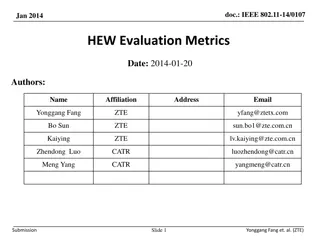
Potential Amendments for Reforming REACH Evaluation Process
Discussion at the Ad-hoc Meeting of Competent Authorities for REACH and CLP focused on potential options for amending the REACH Regulation to reform the evaluation process. Topics included registration-related measures, substance evaluation, testing proposals, compliance checks, and expectations during and after the evaluation process. Proposed measures include adding expiry dates to registrations, strengthening technical completeness checks, and separating registration and evaluation processes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Discussion on potential options for amendments of the REACH Regulation in order to reform REACH evaluation process Ad-hoc Meeting of Competent Authorities for REACH and CLP (CARACAL) 16 March 2022 European Commission & European Chemicals Agency
Agenda WEDNESDAY, 16 MARCH2022 13:30-13:35 1. Adoption of agenda Adoption 2. Registration-related measures Discussion 13:35-14:35 3. Dossier and Substance Evaluation Discussion 14:35-15:20 4. Testing by authorities Discussion 15:20-15:50 Coffee break 15:50-16:05 5. Testing proposal examination Discussion 16:05-16:25 6. Compliance Check Discussion 16:25-16:45 7. Substance evaluation Discussion 16:45-17:05 8. Expectations during (and after) Evaluation process (common provisions) Discussion 17:05-17:35 9. Role of Member State Committee, decision making steps Discussion 16:35-17:50 9. AOB - Reflection on coupling fees with evaluation process 17:50-18:00 10 Summary End of the meeting 17:55-18:00 2
(2) Registration-related measures Ad hoc CARACAL 16 March 2022 Annika Malkia European Chemicals Agency
Contents Expiry of registration Strengthening of technical completeness check Revocation of registration number 4
Registration currently Registration is a licence to market a substance at certain volume and uses. Currently registration licence has no end date; registration number assigned for life . Consequently, the registration is not overturned by: Inactivity following initial registration event Failure to comply with updated information requirements, and improved scrutiny at completeness check Failure to comply with an evaluation decision Current situation does not incentivise maintaining registration aligned with information requirements or contribution to jointly submitted data. 5
Proposed measures: expiry In contrast to current for life status, registrations are given an expiry date by when: Each registrant confirms interest in maintaining the licence to manufacture/import the substance in(to) the EU under a certain scope (volume, uses). ECHA confirms that the registration is renewed if the currently valid registration requirements are met for the scope indicated by the registrant. Failure to take action by the expiry date leads to automatic revocation of the registration. Removal of inactive registrations increases transparency to authorities, JS and stakeholders 6
Proposed measures: stronger TCC Registration and evaluation separate processes; no combination of TCC and CCH Instead, use completeness check to its full potential, to ensure that registration requirements are documented regardless of approach Supports registrants in responsible use of adaptations Ensures approach is properly documented in the dossier > reduces need to clarify grounds for adaptations at CCH Requires clear criteria for elements, e.g. as in Column 2 of Annex VII-X Main improvement area: Annex XI adaptations 7
Proposed measures: revocation In response to persistent non-compliance after CCH, the registration decision, and hence access to the internal market, is revoked: Increased transparency and consistency of consequences of non-compliance Same consequences for all actors: M/I vs. OR Not meant as first option for any delay in responding to evaluation decision Clear and transparent process needed, legitimate expectations & right to be heard Re-registration only after proof of data generation 8
Summary of registration measures EXPIRY REACH evolves, but impact is limited if registrations are not made to follow regulatory developments STRONGER TCC Maximise use of completeness check to ensure any approach to meet information requirements is properly documented in the dossier. REVOCATION Persistent non-compliance wastes authority resources and presents risk for HH/ENV 9
Thank you! name.surname@echa.europa.eu Subscribe to our news at echa.europa.eu/subscribe Follow us on Twitter @EU_ECHA Follow us on Facebook Facebook.com/EUECHA
(3) Dossier and Substance Evaluation Ad hoc CARACAL 16 March 2022 European Chemicals Agency
Compliance Check Objective: Focus on substances of concern Equal playing field? Status post-2027: Expectation: in the context of the integrated regulatory strategy (i.e. grouping): most substances in need of data to conclude on groups have gone through compliance check
Two processes Dossier (DEV) & Substance evaluation (SEV) are fundamentally different processes: DEV against fixed requirements (SIR) in principle suffice to determine RRM SEV addresses concerns beyond SIR Current bottlenecks when combining DEV & SEV DEV should precede SEV Delays possible when SEV detects non-compliance in SIR Also bottlenecks within SEV: Shared roles can lead to sub- optimal efficiency between ECHA and evaluating MSCA Multiple verification steps on multiple versions Different understandings on regulatory science/ decisions Competing resources to ensure timely processing
CARACAL comments Openness to evaluate changing roles for ECHA Request for clear and coherent roles and responsibilities for ECHA and MSCAs Possibility to lower frontier between SEV and DEV e.g. possibility to include CCH requirements in SEV decisions would lead to better synchronisation and complementary of requests Consider options of integrating CCH and SEV for increased efficiency vs running processes in parallel 14
(6) Compliance Check Ad hoc CARACAL 16 March 2022 European Chemicals Agency
Scope of Compliance check Considered extensions (e.g. self-classification, DNEL, CSR/exposure): Objective? Feasibility/Resources? Effectiveness? Impact? Groups and compliance check: Compliance check supports the prioritisation of regulatory needs through the provision of data Grouping substances does not bring or transfer compliance between them
(7) Improving Substance Evaluation (SEV) under REACH Ad hoc CARACAL 16 March 2022 ECHA
Proposals for SEV improvement 1. Clarify the objectives of SEV 2. Increased ECHA involvement 3. No formal CoRAP process
Clarify objectives of SEV (1) Currently a very high burden of proof on authorities, however objectives of REACH to be kept in mind: Existing information leads to uncertainties re. hazards After SEV, a hazard-based concern is clarified and may lead to RMM How to resolve (qualify /quantify) the uncertainties leading to the concern? Do we need scientific data on the substance? Or Can a well-substantiated hypothesis justify to request a study beyond standard information requirements ? Proposal Clarify the burden of proof by authorities on how to establish the concern : 1. Accept that identification of non-hypothetical hazard can originate from: a) substance itself (e.g. in silico models, NAM); b) from similar substance(s) in a group (e.g. Read across, grouping); c) from constituents / metabolites 2. Accept that identification of uncertainty can originate from a substantiated hypothesis 19
Clarify objectives of SEV (2) Do we need to clarify more specifically the hazard concern? Do we need to focus SEV obtain hazard data to clarify the hazard concern? or Do we need to already take exposure into account in SEV, leading to the management of the potential risk (RMM)? Proposal take position on the need for proof of (high) exposure by authorities: Recognize that uncertainty at SEV is hazard- based (also because we often have very little reliable exposure information available in dossiers) Benefits: Facilitate ECHA s grouping approach & allow data generation within the group of similar substances beyond the information on the individual member Avoid (regrettable) substitution within the same group of substances Reduce animal testing if only some substances are tested and conclusion extrapolated on (all) member(s) Allow addressing low tonnage substances (LTS <100tpa) more adequately 20
CARACAL comments General support for hazard-based SEV focus rather than risk-based focus of SEV only Ability to request information on use and exposure, also from downstream users Information on Substance Identity also on Substance Identity Profile Streamline compliance check & SEV major efficiency gains expected (see later) Support framework for SEV evaluation of groups of substances Deadlines for risk management follow-up of SEv conclusions 21
Increased ECHA involvement (1) Starting point: Dossier (DEV) & Substance evaluation (SEV) are fundamentally different processes: DEV outcome should in principle suffice to determine RRM SEV addresses concerns beyond SIR Current bottlenecks when combining DEV & SEV DEV should precede SEV Delays possible when SEV detects non-compliance in SIR Also bottlenecks within SEV: Shared roles can lead to sub- optimal efficiency between ECHA and evaluating MSCA Multiple verification steps on multiple versions Different understandings on regulatory science/ decisions Competing resources to ensure timely processing
Increased ECHA involvement (2) Proposals - two options 1. ECHA is given the possibility to perform SEV (e.g. on request from COM) Still improvements needed on identified hurdles 2. ECHA is given the task to perform all regulatory steps, to increase efficiency & predictability of the process eMSCAs Lead scientific assessment (evaluation and follow-up) Provide scientific support for decision-making and litigations Lead conclusion of SEV + identification of next step(s) ECHA secretariat Regulatory support to eMSCA: GMT/ grouping alignment + coordination (timelines, consistency re. admin. & legal principles + BoA criteria) Take over: drafting and decision making up to sending the adopted decision Ensure publication of SEV conclusions 23
Increased ECHA involvement (3) Current approach Decision- making - FD Data Follow-up Conclusion/ 2ndDD Conclusion next steps CoRAP - Preparation Evaluation - DD Conclusion publication generation - DL eMSCA eMSCA eMSCA eMSCA ECHA Reg. ECHA Future option? Data Follow-up Conclusion/ 2ndDD Conclusion next steps CoRAP - Preparation Drafting DD Decision- making - FD Conclusion publication Evaluation generation - DL eMSCA ECHA ECHA Reg. eMSCA eMSCA ECHA ECHA Reg = registrants
CARACAL comments Openness to evaluate changing roles for ECHA Request for clear and coherent roles and responsibilities for ECHA and MSCAs Possibility to lower frontier between SEV and DEV e.g. possibility to include CCH requirements in SEV decisions would lead to better synchronisation and complementary of requests Consider options of integrating CCH and SEV for increased efficiency vs running processes in parallel Resources needed SEV Conclusions documents: cost-benefits? Re-thinking optimal use of SEV as outcome for RMM! 25
No formal CoRAP process No yearly cycle or process-related (admin.) tasks CoRAP adoption process heavy (with MSC voting) and redundant (never disagreement on the draft) Allow listing (groups of) substances based on the regulatory actions foreseen Alternative to CoRAP RoI for evaluation (based on ECHA s tracking list) Identification of good candidates for SEV occur (mostly) from GMT work/ MSCA s assessment GMT report DEV completed; no RRM process initiated Tracking list 26
CARACAL comments General support for dynamic CORAP Request for lightened process of identification of substances SEv RoI - registry of intentions Need to ensure smooth operation of CoRAP, position of SEv within Integrated Regulatory Strategy providing platform for identification of candidates and concern, & wider context ( one substance-one assessment (1S1A); extension of (P)ACT across chemical sectors) Need for identification and prioritization as well as communication of intentions to stakeholders SEV should be able to start dynamically (short after notification) and no more annual rolling cycle 27
(8) Expectations Ad hoc CARACAL 16 March 2022 European Chemicals Agency
Compliance check: clarify expectations and streamline procedures Clarity of scope Registration as a license Parameters (e.g. tonnage band) and update possibilities Information available and update possibilities Scope of comments by registrant Streamlined procedures: Optimised involvement of MSCAs and MSC Timelines
Thank you! Subscribe to our news at echa.europa.eu/subscribe Follow us on Twitter @EU_ECHA Follow us on Facebook Facebook.com/EUECHA