Chemistry Exam Review: Topics in Scientific Notation, Molecular Weight, Stoichiometry, and Limiting Yield
Explore key concepts in chemistry, including scientific notation, molecular weight calculations, reaction balancing, stoichiometry, and limiting yield problems. Prepare for an upcoming exam by practicing various problems and conversions related to these topics, such as expressing numbers in scientific notation, determining molecular weights of compounds, balancing chemical equations, and solving stoichiometry and limiting yield scenarios.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
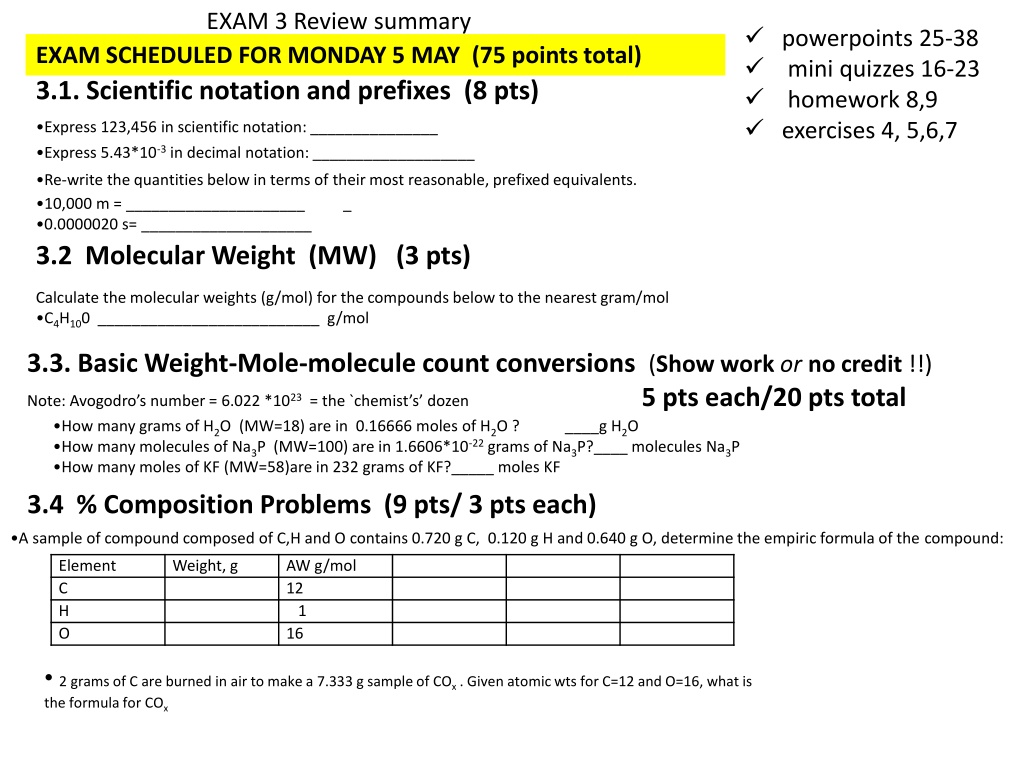
EXAM 3 Review summary powerpoints 25-38 mini quizzes 16-23 homework 8,9 exercises 4, 5,6,7 EXAM SCHEDULED FOR MONDAY 5 MAY (75 points total) 3.1. Scientific notation and prefixes (8 pts) Express 123,456 in scientific notation: _______________ Express 5.43*10-3 in decimal notation: ___________________ Re-write the quantities below in terms of their most reasonable, prefixed equivalents. 10,000 m = _____________________ 0.0000020 s= ____________________ 3.2 Molecular Weight (MW) (3 pts) _ Calculate the molecular weights (g/mol) for the compounds below to the nearest gram/mol C4H100 __________________________ g/mol 3.3. Basic Weight-Mole-molecule count conversions(Show workorno credit !!) 5 pts each/20 pts total Note: Avogodro s number = 6.022 *1023= the `chemist s dozen How many grams of H2O (MW=18) are in 0.16666 moles of H2O ? How many molecules of Na3P (MW=100) are in 1.6606*10-22 grams of Na3P?____ molecules Na3P How many moles of KF (MW=58)are in 232 grams of KF?_____ moles KF ____g H2O 3.4 % Composition Problems (9 pts/ 3 pts each) A sample of compound composed of C,H and O contains 0.720 g C, 0.120 g H and 0.640 g O, determine the empiric formula of the compound: Element C H O Weight, g AW g/mol 12 1 16 2 grams of C are burned in air to make a 7.333 g sample of COx . Given atomic wts for C=12 and O=16, what is the formula for COx
EXAM 3 Review summary (continued) 3.5. Reaction balancing 6 pts (2 pts per equation) Ex. Balance this reaction: ____C8H18 + ___O2-------- _____CO2 + ____H2O 3.6 Chemical Stoichiometry (15 Pts) [Show work for each problem or no credit] 3 problems (avogodro s # =6.022*1023) Example: MW(g/mol) Given the following balanced equation: C3H8 + 5O2 -------- 44 32 44 18 g/mol 3CO2 + 4H2O Compute the number of grams of H2O created by burning 0.06944 moles O2 ? ________g H2O Compute the number of molecules of H2O created by burning 7.30897 g C3H8_______________ MOLECULES Compute the number of moles of CO2 created by consuming 29.333 g C3H8? ______mol C3H8 3.7. Limiting Yield Problems 9 pts (2 problems) 2Al +6HCl 2AlCl3 + 3H2 Ex. MW(g/mol 27 36 133 2 3 mol of Al and 12 moles of HCl are reacted. How many grams of H2 can form? 3.8. T/F (5 pts) The mole concept and dozen concept are essentially the same. Stoichiometry and Souffle recipe calculations are essentially the same. There s nothing special about Avagodro s number. T T T F F F
Chemical Recipe 27 36 133.4 2 g/mol 2Al + 6HCl 2AlCl3 + 3H2 Another limiting reagent problem involving molecule count 27 g Al and 1.0E24 molecules of HCl are reacted. What limits ? HCl How many molecules of H2 can form ? 5*1023
IN-CLASS WORK ON LIMITING YIELD PROBLEMS EXERCISE 7