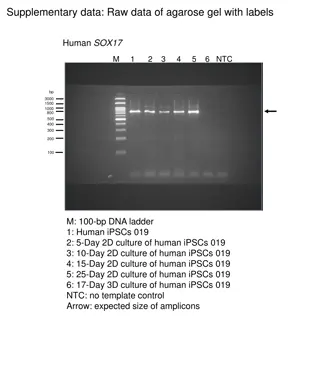
Human Insulin Gene Expression and Production
The process of cloning and expressing the human insulin gene has revolutionized the production of insulin for treating diabetes. By using genetically engineered bacteria, the human insulin gene is inserted, expressed, and purified to create insulin for therapeutic use. This innovation has overcome challenges such as allergic reactions to animal insulin and increased the availability of insulin for patients with diabetes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Cloning and expression of Human Cloning and expression of Human insulin gene insulin gene
Diabetes and the role of insulin Diabetes mellitus Characterized by the excretion of large amount of glucose in urine. Resulted from the body s inability to make sufficient insulin. Insulin A hormone excreted from the cells in the pancreas, and involved in the regulation of blood sugar At high level of blood glucose the insulin is secreted into the bloodstream, circulates throughout the body, and signals the liver and muscle cells to remove the excess glucose.
Diabetes and the role of insulin Insulin recognizes specific insulin receptors on particular cells and initiates a cascade of reactions leading to glucose uptake In absence of glucose uptake, individual cell will starve even plenty of glucose is available Starving cells use fats as a primary source of energy leading to synthesis of ketone bodies The concentration) excreted amount of water. ketone bodies and the glucose with huge (high in urine
Factors led to the development of human insulin production by bacteria The steady increase in the incidence of diabetes Allergic reactions due to animal insulin in some patients Bacterial cells are easy to grow in mass quantity to provides a ready source for product
The first human gene product manufactured by recombinant DNA technology was human insulin, called Humulin, which was licensed for therapeutic use in 1982 by the U.S. Food and Drug Administration (FDA), the government agency responsible for regulating the safety of food and drug products and medical devices. In 1977, scientists at Genentech, the San Francisco biotechnology company cofounded in 1976 by Herbert Boyer and Robert Swanson isolated and cloned the gene for insulin and expressed it in bacterial cells. Genentech, short for genetic engineering technology, is also generally regarded as the world s first biotechnology company.
Steps of insulin production from genetically engineered bacteria 1. Obtain the gene for insulin from human DNA Insert the gene into bacterial cells (restriction endonuclease, vector, transformation) Select the cells that have the desired gene Induce the bacterial cells to express the inserted gene to produce insulin Collect and purify the insulin
Obtaining the insulin gene Finding the insulin gene in the DNA (finding needle in haystack Isolation of mRNA encoding insulin from pancreatic cells mRNA is more abundant in cells than the insulin gene Eukaryotic mRNA contains a poly A tail attached to one end of each RNA This poly A tail can be used to isolate the mRNA from other nucleic acids after breaking the cell
Conversion of mRNA to a complementary strand cDNA by reverse transcriptase enzyme
Problem associated with obtaining the insulin from mRNA It is synthesized by the cells of the pancreas as preproinsulin (inactive) It is stored as proinsulin (inactive) and not released until it is needed by the body Preproinsulin includes the signals necessary to direct the protein to storage. As it is being stored, the part of the protein responsible for signaling storage is specifically removed, leading to formation of proinsulin. Proinsulin is the form that is stored.
Active insulin is formed only when the body in need for insulin then proinsulin is activated by two specific cuts The active form of insulin is actually two separate protein chains (A & B) held together by bonds
Clusters of cells embedded in the pancreas synthesize a precursor polypeptide known as preproinsulin. As this polypeptide is secreted from the cell, amino acids are cleaved from the end and the middle of the chain. These cleavages produce the mature insulin molecule, which contains two polypeptide chains (the A and B chains) joined by disulfide bonds. The A subunit contains 21 amino acids, and the B subunit contains 30. In the original bioengineering process, synthetic genes that encode the A and B subunits were constructed by oligonucleotide synthesis (63 nucleotides for the A polypeptide and 90 nucleotides for the B polypeptide).
Each synthetic oligonucleotide was inserted into a separate vector, adjacent to the lacZ gene encoding the bacterial form of the enzyme b- galactosidase. When transferred to a bacterial host, the lacZ gene and the adjacent synthetic oligonucleotide were transcribed and translated as a unit. The product is a fusion protein that is, a hybrid protein consisting of the amino acid sequence for b-galactosidase attached to the amino acid sequence for one of the insulin subunits The fusion proteins were purified from bacterial extracts and treated with cyanogen bromide, that cleaves the fusion protein from the b- galactosidase. When the fusion products were mixed, the two insulin subunits spontaneously united, forming an intact, active insulin molecule.
Is there a way to get the cells to make active insulin instead of inactive preproinsulin? If each of two chains of the active insulin could be made separately and then properly assembled with the other, active insulin could be produced This is the way recombinant insulin was finally produced. A gene encoding information for one insulin chain was cloned into bacterial cells. The chain was expressed and purified from these cells The information for the other chain was cloned separately, ultimately providing purified second chains
The two strands were mixed outside the cells and bound to each other in proper fashion to form the active insulin molecule Thus, cloned insulin was really produced as a mixture of two separately cloned products.
Because the sequence of amino acids that comprise the two chains was known, this information was used in conjunction with the genetic code to work backward i.e. protein to DNA An advance technique in which fully automated computer process (DNA synthesizer) is finally made it possible to complete the cloning of insulin To obtain insulin genes, the nucleotides sequences of the two chains of the insulin protein are assembled by using DNA synthesizer rather than the mRNA reverse transcription.
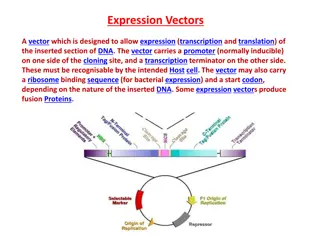
Inserting the insulin gene into bacterial cells The synthesized DNA segments are incorporated within vector eg plasmid The synthesized DNAs do not contain the same recognition site to make the same sticky ends so small artificial pieces of DNA (linkers) that contain the same restriction enzyme recognition sequence as the plasmid are added The linkers are attached to the synthesized DNAs by ligase enzyme then digested with the same restriction enzymes to give the same sticky ends. The vector is then introduced into bacterial cells (competent) by transformation
Inducing the expression of insulin in bacterial cells. Insulin may be degraded by bacterial cells. Once produced by bacterial cells, insulin may be degraded by protease enzyme Use genetically engineered bacteria deficient of protease gene.
Purifying the product (downstream processing)