Clinical Trial Results of Rivaroxaban in ACS Patients: ATLAS-ACS 2 Study
The ATLAS-ACS 2 study investigated the efficacy and safety of rivaroxaban in ACS patients post-index event. The primary endpoints included cardiovascular death, MI, and stroke, with significant reductions seen with rivaroxaban compared to placebo. Stent thrombosis was also reduced with rivaroxaban treatment. Lower doses showed efficacy in reducing all-cause death and cardiovascular events. Overall, rivaroxaban demonstrated benefits in reducing major cardiovascular events in ACS patients.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
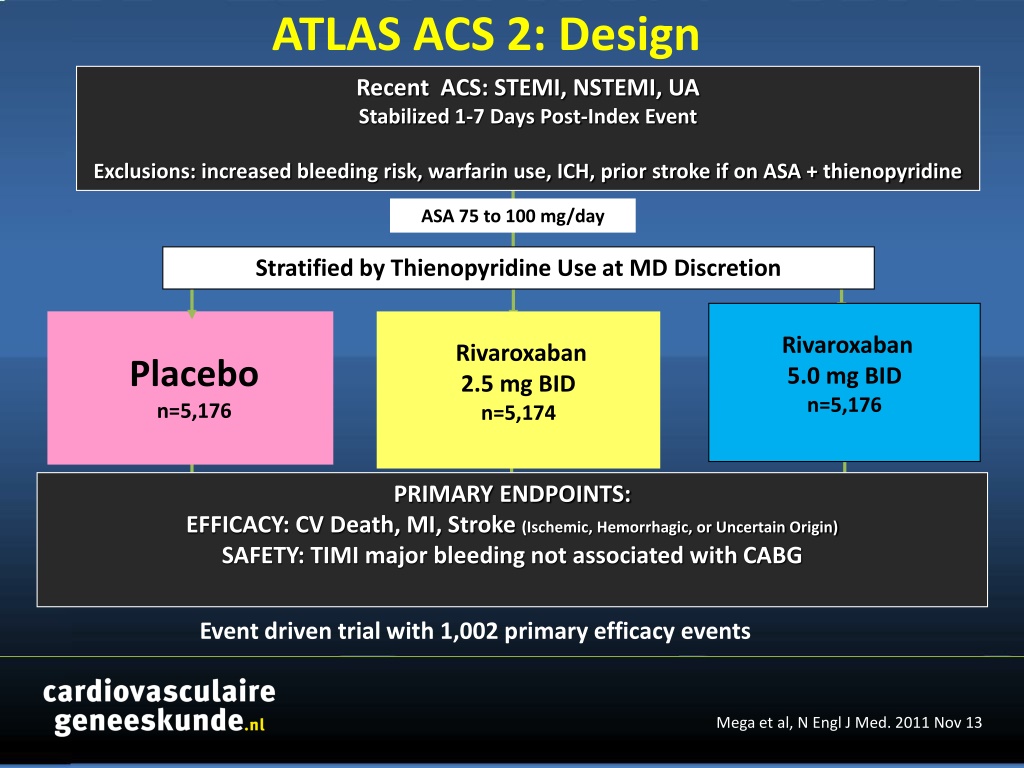
ATLAS ACS 2: Design Recent ACS: STEMI, NSTEMI, UA Stabilized 1-7 Days Post-Index Event Exclusions: increased bleeding risk, warfarin use, ICH, prior stroke if on ASA + thienopyridine ASA 75 to 100 mg/day Stratified by Thienopyridine Use at MD Discretion Rivaroxaban 5.0 mg BID n=5,176 Rivaroxaban 2.5 mg BID n=5,174 Placebo n=5,176 PRIMARY ENDPOINTS: EFFICACY: CV Death, MI, Stroke (Ischemic, Hemorrhagic, or Uncertain Origin) SAFETY: TIMI major bleeding not associated with CABG Event driven trial with 1,002 primary efficacy events Mega et al, N Engl J Med. 2011 Nov 13
ATLAS ACS 2: Baseline Characteristics Placebo Rivaroxaban 2.5 mg BID Rivaroxaban 5.0 mg BID Age, mean (SD) 61.5 ( 9.4) 61.8 ( 9.2) 61.9 ( 9.0) PRE HOSPITAL Sex, male (%) 75.0 74.9 74.2 Prior MI, (%) 27.3 26.3 27.1 Diabetes, (%) 31.8 32.3 31.8 STEMI, (%) 50.9 50.3 49.9 NSTEMI, (%) 25.6 25.5 25.8 UA, (%) 23.6 24.2 24.3 HOSPITAL Revasc at Index, (%) 60.7 60.4 60.4 ASA+Thienopyridine, (%) 93.1 93.3 93.3 Mega et al, N Engl J Med. 2011 Nov 13
ATLAS ACS 2: Primair Eindpunt 2 Yr KM Estimate Placebo 10.7% Geschattecumulatieve incidentie (%) 8.9% HR 0.84 (0.74-0.96) Rivaroxaban (beide doseringen) mITTp = 0.008 ITT p = 0.002 ARR 1.8% NNT = 56 Maanden na randomisatie No. at Risk 5113 4307 3470 2664 1079 1831 421 Placebo Rivaroxaban 10229 8502 6753 5137 2084 3554 831 Mega et al, N Engl J Med. 2011 Nov 13
ATLAS ACS 2: Stent Thrombosis ARC Definite / Probable / Possible 2 Yr KM Estimate 2.9% Placebo Estimated Cumulative Incidence (%) 2.3% HR 0.69 (0.51- 0.93) Rivaroxaban (both doses) mITT p = 0.016 ITT p = 0.008 ARC Definite/probable: HR=0.65, mITT p=0.017, ITT p=0.012 Months After Randomization Mega et al, N Engl J Med. 2011 Nov 13
ATLAS ACS 2: Efficacy endpoints Dose 5.0 mg BID CV Death / MI / Stroke Cardiovascular Death Placebo 10.7% HR 0.94 HR 0.85 Placebo 4.1% 10 Estimated Cumulative Incidence (%) mITT p=0.63 ITT p=0.57 mITT p=0.028 ITT p=0.010 4.0% 8.8% 5 Rivaroxaban 5 mg BID Rivaroxaban 5 mg BID NNT=53 0 24 24 0 0 Months 1 Months Mega et al, N Engl J Med. 2011 Nov 13
ATLAS ACS 2: Efficacy Endpoints Very Low Dose 2.5 mg BID All Cause Death CV Death / MI / Stroke Cardiovascular Death 5% 5% Placebo Placebo HR 0.68 Placebo HR 0.66 12% HR 0.84 4.1% Estimated Cumulative incidence (%) 4.5% 10.7% mITT p=0.002 ITT p=0.004 mITT p=0.002 ITT p=0.005 mITT p=0.020 ITT p=0.007 9.1% 2.9% 2.7% Rivaroxaban 2.5 mg BID NNT = 63 Rivaroxaban 2.5 mg BID NNT = 63 Rivaroxaban 2.5 mg BID NNT = 71 12 12 12 0 24 24 0 0 24 Months Months Months Mega et al, N Engl J Med. 2011 Nov 13
ATLAS ACS 2 Efficacy Endpoints Very Low Dose 2.5 mg BID Patients Treated with ASA + Thienopyridine All Cause Death CV Death / MI / Stroke Cardiovascular Death Placebo 5% 5% Placebo Placebo HR 0.85 HR 0.62 HR 0.64 12% mITT p=0.039 4.5% mITT p<0.001 mITT p<0.001 Estimated Cumulative incidence (%) 10.4% 4.2% 9.0% ITT ITT ITT p=0.011 p<0.001 p<0.001 2.7% 2.5% Rivaroxaban 2.5 mg BID Rivaroxaban 2.5 mg BID NNT = 56 Rivaroxaban 2.5 mg BID NNT = 59 12 NNT = 71 12 12 0 24 24 0 0 24 Months Months Months Mega et al, N Engl J Med. 2011 Nov 13
ATLAS ACS 2: Treatment- Emergent Fatal Bleeds and ICH 1,2 p=NS for Riva vs Placebo p=0.009 for Riva vs Placebo Placebo 2.5 mg Rivaroxaban 5.0 mg Rivaroxaban p=NS for Riva 5 vs Placebo p= 0.005 Riva 5 vs Placebo 1 p=NS for Riva 2.5 vs Placebo P=0.037 for Riva 2.5 vs Placebo p=0.044 for Riva 2.5 vs 5 p=0.44 for Riva 2.5 vs 5 0,8 0,7 0,6 Percent (%) p=NS for all comparisons 0,4 0,4 0,4 0,2 0,2 0,2 0,2 0,1 n=6 n=15 0,1 n=4 0,1 n=5 n=18 n=8 n=14 n=5 n=9 0 Fatal ICH Fatal ICH Mega et al, N Engl J Med. 2011 Nov 13
ATLAS ACS 2: Conclusion Very low dose anticoagulation with rivaroxaban (2.5 mg BID), in addition to antiplatelet therapies, represents an effective strategy to reduce cardiovascular events in patients with a recent ACS. Mega et al, N Engl J Med. 2011 Nov 13