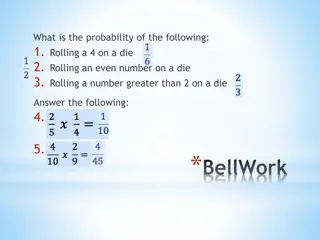Understanding Adverse Events in Clinical Trials
This presentation covers the identification, recording, and reporting of adverse events in clinical trials conducted by University of Edinburgh and/or NHS Lothian. It includes definitions, examples of adverse event recording, SAE form completion, and common mistakes. AEs can range from minor issues like mouth ulcers to serious conditions like heart attacks. The importance of properly documenting and reporting AEs is emphasized to ensure participant safety and trial integrity.
Download Presentation
Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
Presentation Transcript
Identifying, Recording and Identifying, Recording and Reporting Adverse Events in Reporting Adverse Events in Clinical Trials Clinical Trials ACCORD PV Team version 3 1
Before we start Before we start The procedures for identification, recording and reporting of Adverse Events in clinical trials, are laid out in the ACCORD SOP CR005 which can be downloaded from the ACCORD website : http://www.accord.scot/research-access/resources- researchers/sop This SOP applies to all clinical researchers conducting clinical trials sponsored by the University of Edinburgh and/or NHS Lothian. version 3 2
In this presentation In this presentation Identification of an Adverse Event Definitions (slide 5) AE SAE/SAR/SUSARs definitions (slide 7) Example of Adverse event recording (slide 17) How to complete the SAE form and common mistakes (slide 23) version 3 3
ABBREVIATIONS ABBREVIATIONS AE AR SAE SAR SUSAR IMP CTIMP SmPC/ SPC RSI MedDRA IB Adverse Event Adverse Reaction Serious Adverse Event Serious Adverse Reaction Suspected Unexpected SAR Investigational Medicinal Product (ie, a test drug) Clinical Trial of an Investigational Medicinal Product Summary of Product Characteristics Reference Safety Information Medical Dictionary for Regulatory Activities Investigator Brochure version 3 4
Identification of an Adverse Event Identification of an Adverse Event (AE) (AE) An AE is any untoward medical occurrence in a clinical trial participant . So an AE could be as minor as a mouth ulcer, or as significant as a life-threatening heart attack. AEs are not necessarily related to the administration of study drugs; they can arise at any time once a person is deemed to be a participant in a trial. AEs are not always acute events. Diagnoses of osteoarthritis, Crohn s disease, or diabetes, would all comprise AEs. version 3 5
Identification of an Adverse Event Identification of an Adverse Event (AE) (AE) Injuries are also AEs; for example, an accidental scald to the arm while cooking, or a wound to the foot while gardening, would both be considered AEs. Injuries are AEs even if they are self inflicted (either literally, as in deliberate self-harming, or accidentally following drug or alcohol ingestion). Nervous or psychiatric disorders also comprise AEs: dizzy spells, headaches, tremors, the onset of anxiety or depression, psychotic episodes, would all be reportable as AEs. From the preceding examples, it is clear that many scenarios comprising untoward medical occurrences could involve more than one actual AE. We will come back to this point shortly. But first, lets consider how AEs are related to ARs, SAEs, SARs and SUSARs version 3 6
AEs, ARs, SAEs, SARs and SUSARs AEs, ARs, SAEs, SARs and SUSARs Adverse Event: any untoward medical occurrence Not necessarily related to the administration of the IMP AE can be As minor as mouth ulcer / as significant as a life threatening heart attack Acute events or not (diagnoses of osteoarthritis or diabetes are AEs) Injuries (wound to the foot while gardening can be considered as an AE) Self inflicted injuries (either literally or accidentally) Nervous or psychiatric disorders (headaches, anxiety, depression ) version 3 7
AEs, ARs, SAEs, SARs and SUSARs AEs, ARs, SAEs, SARs and SUSARs Adverse Event: any untoward medical occurrence AE Not necessarily related to the administration of the IMP All AEs have to be assessed for : Seriousness Causality Expectedness Severity Assessment of AEs should always be made according to the study protocol version 3 8
AEs, ARs, SAEs, SARs and SUSARs AEs, ARs, SAEs, SARs and SUSARs 1. Seriousness A serious adverse event (SAE) or adverse reaction (SAR) is one that at any dose: Results in death Is life threatening Requires inpatient hospitalisation or prolongation of existing hospitalisation Results in persistent or significant disability or incapacity Consists of a congenital abnormality or birth defect Results in any other significant medical event not meeting the criteria above AE SAE Serious adverse events must be reported to ACCORD immediately or within 24h using the SAE form Email:safety@accord.scot version 3 9
AEs, ARs, SAEs, SARs and SUSARs AEs, ARs, SAEs, SARs and SUSARs 1. Seriousness 2. Causality The Investigator will make an assessment of whether the AE/SAE is likely to be related to the IMP according to the definitions below. AE Unrelated where an event is not considered to be related to the IMP. AR Possibly Related The nature of the event, the underlying medical condition, concomitant medication or temporal relationship make it possible that the AE has a causal relationship to the study drug(s). If considered possibly related the event will be deem an Adverse Reaction (AR or SAR). SAE SAR version 3 10
AEs, ARs, SAEs, SARs and SUSARs AEs, ARs, SAEs, SARs and SUSARs 1. Seriousness 2. Causality 3. Expectedness Only for events assessed as possibly related AE The event may be classed as either: Expected: the AR is consistent with the toxicity of the IMP listed in the RSI (Reference Safety Information). AR SAE SAR Unexpected: the AR is not consistent with the toxicity in the RSI if Serious SUSAR Fatal and life threatening SARs should usually be considered unexpected. Fatal SARs can only be expected for IMPs with an MA in the EU, when it is clearly stated in the list of ARs of the SPC (Section 4.8) that the IMP causes fatal SARs. version 3 11
AEs, ARs, SAEs, SARs and SUSARs AEs, ARs, SAEs, SARs and SUSARs Reference Safety Information A list of expected SARs which are classified using preferred terms according to the Medical Dictionary for Regulatory Activities (MedDRA) Found in the IB in a specific section or in the SPC under Section 4.8 Undesirable Effects Used to assess expectedness where the event is possibly related to the IMP. For CTIMPs, the RSI is approved by the MHRA Sites should always use the approved RSI to assess expectedness Any updates to the IB/SPC will be circulated by the Trial Office There may be more than one RSI if you have more than one medicinal product/intervention in your study version 3 12
AEs, ARs, SAEs, SARs and SUSARs AEs, ARs, SAEs, SARs and SUSARs Examples of RSIs For products with a marketing authorisation, the RSI is often section 4.8 of the approved Summary of Product Characteristics (SPC) For products without a marketing authorisation, the RSI is normally contained within a specific section of the Investigators Brochure (IB) For Medical Devices, the risk analysis report is often used as the source of expected events. Example of a Table from section 4.8 Undesirable effects version 3 13
AEs, ARs, SAEs, SARs and SUSARs AEs, ARs, SAEs, SARs and SUSARs 1. Seriousness 2. Causality 3. Expectedness AE Expectedness assessment only required if event is assessed as possibly related AR Compare the AR to the approved RSI. If the AR is: - Not listed in the RSI - Listed in the RSI but more severe, common or different in nature to the listed reaction Then it is an UNEXPECTED event SAE SAR SUSAR SUSAR Suspected unexpected serious adverse reaction version 3 14
AEs, ARs, SAEs, SARs and SUSARs AEs, ARs, SAEs, SARs and SUSARs SUSAR: Any AR which is: Classified as serious Suspected to be caused by the IMP Unexpected because the event is not consistent with the RSI/SPC SUSARs are reported by ACCORD to the MHRA and REC for CTIMPs studies Incorrect assessment of expectedness can lead to under reporting of SUSARs which could potentially put patients at risk Note: for blinded studies we refer to potential SUSARs until the Sponsor unblinds the participant s treatment and confirms they received active IMP. Any such confirmation will not be revealed to the blinded investigators. Blinded researchers will also not be told if a SUSAR is onward reported to the MHRA and REC (again, in order to maintain the blinding). version 3 15
AEs, ARs, SAEs, SARs and SUSARs AEs, ARs, SAEs, SARs and SUSARs 1. Seriousness 2. Causality 3. Expectedness AE 4. Severity Severity: Mild or moderate or severe depending on tolerability of the event, level of discomfort caused and the level the event interferes with every day activities. AR SAE SAR SUSAR The term severe should not be confused with term serious version 3 16
Example of Adverse Events Example of Adverse Events recording recording Event: A participant on a study was reported to have had panic attacks, hallucinations, nausea, dizziness and drowsiness, and finally a loss of consciousness. If it is possible for the PI at the site to make a single, definitive, diagnosis based on this report, then that single diagnosis can be recorded as a single AE. If it is NOT possible to make a single, definitive diagnosis, then each item in the description that comprises a recognised MedDRA preferred term (PT) must be recorded as a separate AE. version 3 17
Example of Adverse Events Example of Adverse Events recording recording Adverse events identified by the study site should be entered into the AE log. One event per line version 3 18
Example of Adverse Events Example of Adverse Events recording recording version 3 19
Example of Adverse Events Example of Adverse Events recording recording 1 SUSAR 2 SAEs 3 AEs version 3 20
Example of Adverse Events Example of Adverse Events recording recording MedDRA Coding to SOC level is mandatory for all the studies recorded in EudraCT version 3 21
Example of Adverse Events Example of Adverse Events recording recording The two SAEs identified on the AE log should also be recorded on a SAE form. A new form should be completed for each of the SAEs. Please make sure you don t include any patient identifiable data other than the participant s number in the SAE form, in other attachments or in the email to the PV Team. version 3 22
Example of Adverse Events Example of Adverse Events recording recording the SAE form the SAE form Once PI is made aware that a SAE occurred 24h to provide a pdf copy of the SAE form to the Sponsor safety@accord.scot even if it is not possible to fully complete the form. Investigators should submit updated forms as and when any missing information becomes available. The same form used for the initial submission should be updated with the missing information do not start a new form. version 3 23
Example of Adverse Events Example of Adverse Events recording recording the SAE form the SAE form New observations that are considered to be part of a previously reported but ongoing single definitive diagnosis (that has already been reported as such) should be included as updated information in the original SAE form. If additional AEs/SAEs subsequently become apparent for the same participant, they should be documented as separate entries in the AE log, and submitted on new SAE forms. Assessment of AEs/SAEs should always be made according to the study protocol some events might not be reportable according to the protocol version 3 24
Example of Adverse Events Example of Adverse Events recording recording the SAE form the SAE form The Date of onset is the Date of the first signs and/or symptoms of the event (and not the date the event became serious) version 3 25
Example of Adverse Events Example of Adverse Events recording recording the SAE form the SAE form The Diagnosis field in the form should state the main event, the overall diagnosis to which the seriousness criteria applies If it is possible, the PI at the site should make a single, definitive, diagnosis, If it is NOT possible to make a single, definitive diagnosis, then each item in the description that comprises a recognised MedDRA preferred term (PT) and meet the seriousness criteria must be recorded as a separate SAE. If the diagnosis or event description has changed as a result of follow-up, then causality and if necessary expectedness should be reassessed by the site PI. version 3 26
Example of Adverse Events Example of Adverse Events recording recording the SAE form the SAE form Event Description: Please try to limit the use of abbreviations here and in the diagnosis field If any symptom or event mentioned in the Description of SAE field meets seriousness criteria in its own right, please complete a separate SAE form for this event If any symptom mentioned in the Description of SAE field is not a symptom of the overall Diagnosis , and does not meet serious criteria, this should be recorded as a separate AE in the AE log version 3 27
Example of Adverse Events Example of Adverse Events recording recording the SAE form the SAE form Provide at least one seriousness criteria If other seriousness criteria applies (Please note that New events/reactions likely to affect the safety of participants means it has an implication for the other participants of the study. If ticked the report will have to be reported to Health Authorities) All the sections and fields of the SAE form have to be filed for the form to be considered completed. PV Team will follow-up with site team until the form is completed. version 3 28
Example of Adverse Events Example of Adverse Events recording recording the SAE form the SAE form Tick YES if participant received the IMP at ANY point before the SAE occurred version 3 29
Example of Adverse Events Example of Adverse Events recording recording the SAE form the SAE form Provide the Causality and ALWAYS provide a Rationale for the Causality Assessment The causality assessment (and expectedness assessment if required) should be made against the Diagnosis event version 3 30
Example of Adverse Events Example of Adverse Events recording recording the SAE form the SAE form Expectedness section to be completed for possibly related reports ONLY The expectedness assessment should be made against the Diagnosis event version 3 31
Example of Adverse Events Example of Adverse Events recording recording the SAE form the SAE form The PI should use the RSI to assess if the event is expected or unexpected. The version* of the SmPC or IB used has to be indicated. Only the RSI that is authorised by the MHRA at the time the potential SAR/SUSAR occurred (onset date) can be used for the expectedness assessment. Previously circulated versions that have been superseded must not be used *The version of the RSI for a SPC is not the date of the SPC booklet but can be found in section 10 - Date of revision of the text version 3 32
Example of Adverse Events Example of Adverse Events recording recording the SAE form the SAE form Sections 4 to 7: Complete or tick no Section 8: complete and don t forget to add initials and date in the required column Section 9: complete and don t forget to add initials and date in the required column The PV Team will follow-up with site team until a final outcome is provided Section 11 to 13: each form (initial and follow up forms) has to be signed and dated by the PI Section 5 is to indicate the concomitant medication RELEVANT to the SAE. Do not include the treatment used to treat the SAE version 3 33
Resources SOPs | Accord SOP CR005 Identifying, Recording and Reporting Adverse Events and Urgent Safety Measures for CTIMPs Forms: CTIMPS AE log CTIMPS SAE form Pregnancy notification form Parent Child SAE form version 3 34
If you have any question or issue If you have any question or issue that are AEs/SAEs related, please that are AEs/SAEs related, please don t hesitate to contact us: don t hesitate to contact us: safety@accord.scot version 3 35