Overview of Secondary Metabolites: Alkaloids, Flavonoids, Steroids, and Glycosides
Exploring the diverse world of secondary metabolites, this content delves into the composition, chemistry, biosources, therapeutic uses, and commercial applications of various compounds such as Alkaloids, Flavonoids, Steroids, Glycosides, and more. It discusses the structural characteristics, biological significance, and classification of these secondary metabolites, shedding light on their roles in plant growth, pigmentation, and enzymatic activities.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Anju Singh School of Pharmaceutical Sciences, CSJM University, Kanpur 1
UNIT-II General introduction, composition, chemistry & chemical classes, biosources, therapeutic uses and commercial applications of following secondary metabolites: Alkaloids: Vinca, Rauwolfia, Belladonna, Opium, Phenylpropanoids and Flavonoids: Lignans, Tea, Ruta Steroids, Cardiac Glycosides & Triterpenoids: Liquorice, Dioscorea, Digitalis Volatile oils : Mentha, Clove, Cinnamon, Fennel, Coriander, Tannins: Catechu, Pterocarpus Resins: Benzoin, Guggul, Ginger, Asafoetida, Myrrh, Colophony Glycosides: Senna, Aloes, Bitter Almond Iridoids, Other terpenoids & Naphthaquinones: Gentian, Artemisia, taxus, carotenoids 2
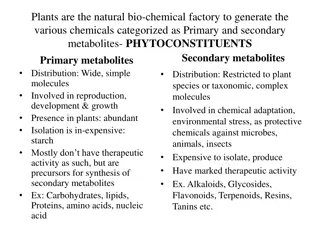
Second important class of the biphenylpropanoid derivatives is known as the Flavonoids. polyphenolic compounds which are found in fruits, flowers, seeds & vegetable. 2000 different compounds reported to be present mostly phenols either in the free state or as the glycosides flavous is a latin word yellow colour (yellow-coloured compounds) They have a very limited role in this respect due to their low toxicity when compared with other plant secondary metabolites such as alkaloids. They are the pigments of flowers and attract pollinating insects. They play a role in plant growth control by inhibiting & activating enzymes. 3
The flavonoids possess15 carbon atoms; two benzene rings joined by a linear three carbon chain the skeleton can be represented as the C6 - C3 - C6 carbon skeleton having a pyran or chroman ring second benzene (aromatic) ring strategically positioned at C 2, C 3 or C 4 The three-carbon (-C3-) may be included through an oxygen bond between the two phenyl rings into: 1- A 5-membered heterocyclic ring (furan) as in aurones 2- A 6-membered heterocyclic ring (pyran) to give flavonoids constitute the largest group. 3' 4' 1 6 4' 3' 3' 2' 4' 2' 5' 2' B 1 8 1' 7 3 5' B O 7 O 6' 2 4 C 2 5' 6' C A A 6' 2 6 3 5 5 C 3 4 5 4 C 6 4
flavones, flavanones, flavonols, isoflavones, and Anthocyanidins: colored aglycones found as a large number of pigments from flower and fruits In certain specific cases either the 6-membered heterocyclic ring (pyrones) is replaced by 5 membered heterocyclic ring (aurones) or exists in an open-chain isomeric form (chalcones) Apart from glycosylated derivatives, methylated, acetylated, prenylated, or sulphated derivatives also exist Flavonoid aglycone : consists of benzene ring A, condensed with 6 membered ring C pyran ring, which in 2nd position carries phenyl ring B as a substituent 3' 2' 4' B 1 8 1' 5' O 7 2 6' C A 6 3 5 4 5
The flavonoid glycosides: Glycosides aglycone (non sugar part)+glycone (sugar part) When glycosides are formed, the glycosidic linkage can be located in positions 3 or 7 and may be L-rhamnose, D- glucose, galactose or arabinose 3' 2' 4' B 1 8 1' 5' O 7 2 6' C A 6 3 4 5 6
Based on the carbon of the C ring on which B ring is attached, and the degree of unsaturation and oxidation of the C ring. Flavonoids in which B ring is linked in position 3 of the ring C are called isoflavones; Those in which B ring is linked in position 4 neoflavonoids Flavonoids with open C ring are called chalcones. Those in which the B ring is linked in position 2 further subdivided into several subgroups on the basis of the structural features of the C ring. These subgroup are: flavones, flavonols, flavanones, flavanonols, flavanols or catechins and anthocyanins. 7
O Have a double bond b/w positions 2 and 3 and a ketone in position 4 of the C ring. Most flavones of vegetables and fruits has a hydroxyl group in position 5 of the A ring, while the hydroxylation in other positions, for the most part in position 7 of the A ring or 3 and 4 of the B ring may vary according to the taxonomic classification of the particular vegetable or fruit. Glycosylation occurs primarily on position 5 and 7, methylation and acylation on the hydroxyl groups of the B ring. Some flavones, such as nobiletin and tangeretin, are polymethoxylated. O 8
O OH O Compared to flavones, they have a hydroxyl group in position 3 of the C ring, which may also be glycosylated. Again, like flavones, flavonols are very diverse in methylation and hydroxylation patterns as well, and, considering the different glycosylation patterns, they are perhaps the most common and largest subgroup of flavonoids in fruits and vegetables. For example, quercetin is present in many plant foods. 9
O Flavanones (dihydroflavones) Have C ring saturated; unlike flavones, the double bond between positions 2 and 3 is saturated. the only structural difference between the two subgroups of flavonoids. The flavanones can be multi-hydroxylated, and several hydroxyl groups can be glycosylated and/or methylated. Some have unique patterns of substitution, example: furano-flavanones, prenylated flavanones, pyrano- flavanones or benzylated flavanones, giving a great number of substituted derivatives. O 10
Flavanonols Flavanonols, also called dihydroflavonols, are the 3-hydroxy derivatives of flavanones; they are an highly diversified and multisubstituted subgroup. Isoflavones As anticipated, isoflavones are a subgroup of flavonoids in which the B ring is attached to position 3 of the C ring. They have structural similarities to estrogens, such as estradiol, and for this reason they are also called phytoestrogens. Neoflavonoids They have the B ring attached to position 4 of the C ring. O O FLAVANONOLS O O Neoflavonoids OH Isoflavones O 11 O
Flavanols or flavan-3-ols or catechins Flavanols are also referred to flavan-3-ols as the hydroxyl group is almost always bound to position 3 of C ring; they are called catechins as well. flavanols to have two chiral centers in the molecule, on positions 2 and 3, then four possible diastereoisomers. Epicatechin is the isomer with the cis configuration and catechin is the one with the trans configuration. Each of these configurations has two stereoisomers, namely, (+)- epicatechin and (-)-epicatechin, (+)-catechin and (-)- catechin. (+)-Catechin and (-)-epicatechin are the two isomers most often present in edible plants. FLAVANOLS O 12
Another important feature of flavanols, particularly of catechin and epicatechin, is the ability to form polymers, called proanthocyanidins or condensed tannins. The name proanthocyanidins is due to the fact that an acid-catalyzed cleavage produces anthocyanidins. Proanthocyanidins typically contain 2 to 60 monomers of flavanols. Monomeric and oligomeric flavanols (containing 2 to 7 monomers) are strong antioxidants. 13
Anthocyanidins: Chemically, anthocyanidins are flavylium cations and present as chloride salts. Only group of flavonoids that gives plants colors (all other flavonoids are colorless). Anthocyanins are glycosides of anthocyanidins. Sugar units are bound mostly to position 3 of the C ring and often conjugated with phenolic acids, as in ferulic acid. The color of anthocyanins depends on pH & also by methylation or acylation at the hydroxyl groups on the A and B rings. Chalcones Chalcones and dihydrochalcones are flavonoids with open structure; they are classified as flavonoids because they have similar synthetic pathways. OH * O O 14
If we consume food containing lignan precursor, it is changed into enterolignans, enterodiol, enterolactone by bacterial action residing in the colon enterodiol, enterolactone have weak estrogenic activity & may exert action by non estrogenic mechanism enterodiol, enterolactone can mimic some actions of estrogens- so plant derived lignan precursor are called as Phytoestrogens HO HO OH O OH ENTEROLACTO NE O ENTERODIOL OH OH 15
1948- Haworth- introduced term- Lignan It is grp of dimeric phenylpropenoid Lignans subgroup of non flavanoid polyphenols Based on C skeleton, cyclization pattern and the way O is added in the skeleton they are divided into 8 types 1. Furofuran 2. Furan 4. dibenzylbutyrolactol 5. dibenzylbutyrolactone 6. Aryltetralin 7. Arylnaphtalene 8. dibenzocyclooctadienes 3. Dibenzylbutane 16
de la Rosa L.A., Alvarez-Parrilla E., Gonzlez-Aguilar G.A. Fruit and vegetable phytochemicals: chemistry, nutritional value, and stability. 1th Edition. Wiley J. & Sons, Inc., Publication, 2010 Han X., Shen T. and Lou H. Dietary polyphenols and their biological significance. Int J Mol Sci 2007;9:950-988. doi:10.3390/i8090950 Manach C., Scalbert A., Morand C., R m sy C., and Jime nez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79(5):727-47 doi:10.1093/ajcn/79.5.727 Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010;2:1231-1246. doi:10.3390/nu2121231 17