Estimation of Alkalinity in Water Sample Practical for B.Sc. III Year
Alkalinity in water samples can be estimated by titrating with HCl and using indicators like Phenolpthalein and Methyl orange. The presence of OH-, CO3-, and HCO3- contributes to alkalinity, giving pink or yellow colors with different indicators. The process involves adding indicators, titrating with HCl, and calculating the alkalinity concentration in mg/L. This practical session presented by Manoj Joshi covers the reagents, procedure, observation table, and calculations involved in determining alkalinity in water samples.
- Alkalinity estimation
- Water sample analysis
- B.Sc. practical
- Titration method
- Water quality assessment
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Estimation of Alkalinity in given Water Sample Class- B.Sc. III Year Practical Date: 14.10.2020 Time: 03:00-05:00PM Presented by Manoj Joshi Department of Zoology UCoS,MLSU, Udaipur.
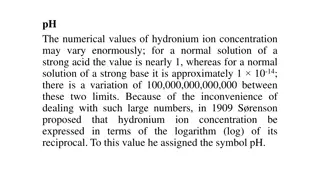
Alkalinity in water is due to presence of OH-, CO3- and HCO3-. Alkaline water gives yellow colour with methyl orange indicator and pink colour with Phenolpthalein indicator. Apart from these in colourful water in natural conditions humic acid and fulvic acid gives alkalinity. If in the given sample Carbonate is present then it has pH 8.3 and by adding Phenolpthalein indicator it shows pink colour. If methyl orange indicator is added then sample contain yellow colour with is converted in pink colour while it is titrated with acid.
Reagents:- i. o.1 N HCl:- 34ml HCl + 100 ml DW. ii. Methyl orange indicator iii. Phenolpthalein indicator. Procedure:- Take 50 ml sample in a conical flask. Add 2 drop of Phenolpthalein indicator if it gives pink colour then titrate with HCl till disappear of colour and note the reading. Add 2 drop of Methyl orange indicator and titrate with HCl till pink colour disappearance and note the reading. Repeat the process 3 time for better result.
Observation table:- Burette Reading Sample in ml S. No For Methyl orange indicator For Phenolpthalein indicator Start End Total (A) Start End Total (B) 1 2 3
Calculation:- PA = A x N of HCl X 1000 x 50 ml of sample = A x 0.1 x 1000 x 50 50 = A x 100 mg/L. TA = B x N of HCl X 1000 x 50 ml of sample = B x 0.1 x 1000 x 50 50 = B x 100 mg/L.
Thank You