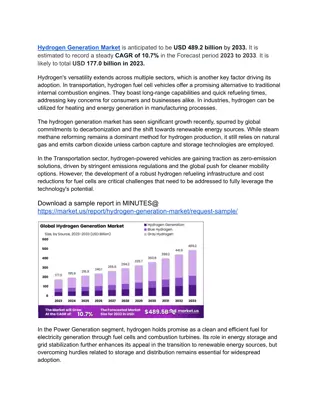
Gas Detection of Hydrogen/Natural Gas Blends in the Gas Industry
Gas detection instruments play a crucial role in assessing the presence of hazardous atmospheres in the gas industry. This study focuses on the impact of adding hydrogen up to 20% in natural gas blends on gas detection instruments. The aim is to understand any potential inaccuracies in readings and determine measures for ensuring accuracy. The research explores detector types, sensor mechanisms, target gases, and solutions for accurate gas detection in the future.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Gas detection of hydrogen/ natural gas blends in the gas industry ID128 26/09/2019 J Hall, K Jeffrey, P Hooker
Contents Introduction to gas detection in the gas industry Instrument testing CO response Flammable PPM response Flammable LEL response Flammable volume % response O2 depletion response Impact of findings on HyDeploy Solutions to be employed at Keele University Summary of detector requirements for the future
Gas detection Gas detection instruments are widely used to assess the presence of dangerous atmospheres The way these are used varies by GDN Any changes due to gas composition will most likely affect procedures rather than the QRA
Aims The aim of the pre-deployment phase of HyDeploy for gas detection was to understand: 1. If the addition of H2 up to 20 vol% in NG will cause current gas detection instruments to display uncorrected readings 2. What measures might be needed to ensure accurate readings
Detector types Two basic categories: 1. PORTABLE instruments, which can be subdivided into: survey instruments intended for quantitative assessment and location of gas escapes personal safety monitors intended to protect staff from dangerous atmospheres 2. FIXED instruments, which can be subdivided into: domestic monitors intended for use in the home with the public plantroom monitors intended to provide multiple alarm levels and to isolate gas supplies at known set points
Sensor mechanisms Commercially available gas sensors are based on several sensor mechanisms Catalytic Thermal conductivity Semi-conductor Flame ionisation detector Electrochemical Infra-red adsorption Palladium H sensors
Detector target gases Generally, each gas detection instrument will target multiple gases which may cause harm: CO Flammable ppm range (NG) Flammable LEL range (NG) Flammable volume % range (NG) O2 deficiency
Testing scope Test results and issues are split by gas type and range: 1. CO detectors response 2. Flammable detector response i. ppm range ii. LEL range iii. volume % range 3. O2 depletion response
Test methods Two test methods were used: 1. Exposure to ppm levels of H2 To understand the concentration of H2 required to trigger nuisance alarms on CO detectors Provides data on flammable response at the ppm range A further test was also performed using 203 ppm CO in air to assess any potential cross-sensitivity 2. Exposure to flammable blends from LEL to volume range Assess the effect of blended mixtures of H2 and CH4 in air across a range of blend concentrations Generate a complete dataset across both measurement ranges
PPM H2 test set-up (1) Flow controllers to dilute span gasses (accurate to 0.1% of full scale) Rotameter fitted to the end of the test pipe to verify positive flow, i.e. no air ingress Manifold for test instruments Calibrated span gasses certified mixtures of CO/H2 in air
Flammable blend test set-up (2) Rotameter fitted to the end of the test pipe to verify positive flow, i.e. no air ingress Manifold for test instruments
Test concentrations and volume fractions for tests ppm H2 testing Flammable blend testing Applied flammable fraction (vol%) Applied flammable fraction (% LEL, assuming 4.4 vol% for CH4) Applied H2 conc. (ppm) 0 40 80 120 160 200 400 600 Span flow rate (cc/min) 0 50 100 150 200 250 500 750 Air flow rate (cc/min) 10000 9950 9900 9850 9800 9750 9500 9250 0 0.0 6.0 11.9 17.9 23.8 47.6 71.4 95.2 0.25 0.5 0.75 1 2 3 4 20 40 60 80 100 LEL range Volume range NA Repeated for blends of 0, 10, 15 and 20 vol% H2 in CH4 All test ranges were repeated a further two times, i.e. three data points per concentration
Summary - CO detector response CO detectors are cross-sensitive enough to H2 that even the maximum permitted leak rate might trigger a false CO alarm Some flammable detectors are cross-sensitive to CO No O2 detector tested showed any cross-sensitivity to ppm levels of CO or H2 All CO detectors saturated when exposed to high levels (LEL) of blend gas To be expected based on cross sensitivity observed during LEL level tests Detectors required recovery times in hours before passing a bump test No long-term desensitisation was observed after 24 hours in clean air Exact time taken to normalise was not determined
Summary - flammable PPM response Relative response (Applied H2 concentration / flammable ppm response) 0.46 0.86 1.13 0.59 Over-range level, H2 (ppm) A C B D 120 >600 >600 >600 Results show that all flammable gas detectors respond to H2 The relative response figure gives a measure of how responsive the given detector is to H2 relative to CH4 <1 : detector is less sensitive to H2 than CH4 =1 : detector is as sensitive to H2 as CH4 >1 : detector is more sensitive to H2 than CH4
Summary - flammable LEL response Testing showed that all detectors LEL outputs are offset by exposure to the blend gas rather than CH4 All catalytic based detectors exhibit a relative increase in instrument output when exposed to H2 The IR detector exhibited a relative decrease in instrument output proportional to H2 concentration in the blend All detectors outputs remained linear regardless of blend concentration Therefore a correction factor can be used convert the measured output into an accurate/actual value
Correction factor components 1. Blend factor LEL physically changes with the addition of H2 to CH4 for blended mixtures H2 LEL = 4.0 vol%, CH4 LEL = 4.4 vol%, NG LEL = 5.0 vol% Higher H2 content in blend decreases overall LEL, at 20 vol% H2 in CH4 the LEL is 4.31 vol% based on Le Chatelier correlation 2. Calibration/ranging factor Different instruments are calibrated to different LEL ranges (either 4.4% or 5%)! Although GDN standard for procedural development is 5% Gas used by manufacturers and HSL for calibration may different correction factor based on HSL calibration using CP grade CH4 (i.e. LEL 4.4 vol%) 3. H2 cross sensitivity factor The instruments sensors are all affected differently by the H2 content of the blend either due to differing sensor technologies (e.g. catalytic vs. IR) or inherent differences in the design/implementation of the technology If all of the instruments were ranged and calibrated the same and the H2 content of the blend is known, the H2 factor is only remaining variable between devices
Flammable LEL response correction factor Blend ratio of H2 in CH4 (vol%) Blend correction factor Instrument Response Final correction factor Instrument model 1.000 1.009 1.016 1.020 1.000 1.009 1.016 1.020 1.000 1.009 1.016 1.020 1.000 1.009 1.016 1.020 1.000 1.009 1.016 1.020 1.000 0.947 0.954 0.938 1.000 0.977 0.994 0.922 1.000 0.974 0.978 0.951 1.000 1.149 1.147 1.282 1.000 1.014 0.972 0.957 1.000 0.956 0.970 0.958 1.000 0.986 1.010 0.941 1.000 0.983 0.994 0.971 1.000 1.160 1.165 1.309 1.000 1.023 0.987 0.977 0 10 15 20 0 10 15 20 0 10 15 20 0 10 15 20 0 10 15 20 A B Actual LEL = Instrument reading * Correction factor C D E Example If instrument A was used to measure the LEL of a 20% blended gas mixture at 2.00 vol% in air it would give an instrument reading of 42.6% LEL (based on test data) In order to get the corrected value and hence the actual % LEL the instrument reading must be multiplied by the corresponding final correction factor to get 42.6% LEL * (0.958) = 40.8% LEL
Summary - flammable vol% response Testing showed that all detectors vol% outputs are offset by exposure to the blend gas rather than CH4 All thermal conductivity based detectors exhibit a relative increase in instrument output when exposed to H2. Thermal conductivity detectors outputs fit a second order polynomial when H2 blends are introduced The IR detector exhibited a relative decrease in instrument output proportional to H2 concentration in the blend A more complicated correction factor is needed for thermal conductivity based detectors IR detectors outputs remained linear therefore a simplistic linear correction factor can be used
Flammable vol% response correction factor Example instrument output at applied gas concentration of 50 vol% 51 68 Instrument model Blend ratio of H2 in CH4 (vol%) To calculate the actual level from a reading Instrument correction factor 0 10 0.984x 0.00008x2+0.7455x -0.0007x2+0.7242x -0.0006x2+0.6637x 0.9914x -0.0015x2+0.9275x -0.0025x2+0.9198x -0.0026x2+0.8787x 0.982x -0.0022x2+1.0269x -0.0049x2+1.1565x -0.0051x2+1.1309x 0.9697x 1.0668x 1.1356x 1.1982x Use the instrument correction factor equation where x is the reading A 15 74 20 0 10 81 51 60 B 15 67 20 0 10 74 51 56 C 15 59 20 0 10 15 20 65 52 47 44 42 D Example If instrument A was used to measure the 20% blended gas mixture at 50 vol% in air it would give an instrument reading of 81% In order to get the corrected value and hence the actual % the instrument reading must be substituted into correction factor equation to get - 0.0006*(812)+0.6637*81 = 49.8%
Summary oxygen depletion response Results from testing of the O2 detectors with flammable blends across the PPM, LEL and vol% ranges showed little-to-no cross-sensitivity to blended mixtures of H2 and CH4 The only instrument to show any consistent deviation was the 7B-500R and this only deviated by a maximum of -1 vol% across the scale
Scenarios Scenario Issue for current detectors? Indoor CO leak No Indoor leak of blended gas at current permissible leakage rates Possibly, false CO alarms may occur although its difficult to estimate dispersion, room volumes and air change rates Indoor leak of blended gas up to evacuation level (20% LEL) Yes, cross-sensitivity of CO detectors to blends will cause false alarms/evacuations Indoor leak of blended gas and CO Yes, can t distinguish between them so false alarms/evacuations may occur Outdoor leak of blended gas or NG Possibly, blend percentage won t be known so difficult to get accurate instrument reading but will get a detect/no-detect reading Confined space entry No Purging of pipework Yes, incomplete purging from air to gas may occur due to blend increasing instrument reading
Indoor leak of blended gas at current permissible leakage rates false CO alarm possibility CO Trigger Action Level (ppm)1 10 30 200 10 30 200 A large well-ventilated room2 A small poorly-ventilated room3 A C B E Red fill indicates a false alarm is possible Green fill indicates a false alarm is unlikely 1CO exposure limits: 200 ppm (evacuate), 30 ppm (force entry after letterbox test), 10 ppm (re-occupy) 2A large well-ventilated room, with a volume of 22 m3, a ventilation rate of 2.5 air changes per hour (ach) and a maximum NG leakage rate of 0.011 m3/hr would give rise to a maximum homogeneous H2 concentration of 40 ppm (200 ppm of H2/CH4 for a 20% blend) 3A small poorly-ventilated room, with a volume 12.4 m3, a ventilation rate of 0.5 ach and a maximum NG leakage rate of 0.0062 m3/hr would give rise to a maximum homogeneous H2 concentration of 200 ppm (1000 ppm of H2/CH4 for a 20% blend).
Indoor leak of blended gas at current permissible leakage rates false CO alarm possibility CO Trigger Action Level (ppm)1 10 30 200 Concentration of 20 vol% H2 in CH4 blend gas to trigger alarm (ppm)2 202 469 278 830 101 283 274 746 A C B E 2740 5524 1832 4756 Leak is below effective odorant level3 and may not be smelt Leak is above effective odorant level3 and should be smelt 1CO exposure limits as set out in EH40: 200 ppm (evacuate) 30 ppm (force entry after letterbox test) 10 ppm (re-occupy) 2Assuming a 20% H2 to NG blend with no effect of NG on the CO detector i.e. no cross sensitivity to NG, only H2 3The effective odorant level requires an odour intensity of 2 (I.e. olfactory degree providing 99% certainty of detection) at 20% LEL for NG (1% NG in air). This equates to a H2 concentration level (20% blend in NG) of 1%x20% = 0.002% or 2000 ppm.
Summary Each make/model of detector showed consistent results across the 3 duplicate devices tested Multiple instruments/readings were found to be cross sensitive to H2/CH4 blends. This is summarised in the table below for blends up to 20% Flammable PPM detector Flammable LEL detector Flammable vol% detector O2 depletion detector Instrument CO detector A C B E D Detector not fitted in this instrument Cannot distinguish H2 Significant cross-sensitivity to blends which will need to be mitigated No/insignificant cross-sensitivity
Solutions to be employed at Keele University flammable leak detection Use an IR based gas detector calibrated for a worst case blend concentration of 20 vol% H2 in NG Always conservative but may evacuate earlier with lower blends Thermal conductivity based detectors over-read with H2 and would hence not be conservative when measuring flammables Thermal conductivity based detectors would need to independently identify H2 composition of gas to get correct flammable reading
Solutions to be employed at Keele University purging operations Use bottled NG when purging operations are required, not ideal long term Alternative procedure based on a relative 90% GIA reading under consideration Thermal conductivity based detectors would need to compensate for blend concentration to be accurate and would need a way of measuring H2 independently IR based detectors calibrated for NG would never reach 90 vol% gas in air with a blend If IR detectors calibrated for a worst case blend (20 vol% H2) when a lower blend/NG is used, a reading of 90% would be obtained prematurely Any detection instrument would need to independently identify H2 composition of gas to get correct flammable reading
Whats needed going forward? A CO detector which can be incorporated into a portable instrument which is not sensitive or sufficiently compensated to H2 If such detectors cannot deal with vol% levels of H2 then the CO detector should be automatically isolated during measurement modes where high levels of blend may be encountered A domestic fixed CO detector which is not sensitive or sufficiently compensated to H2 Flammable detectors across the ppm, LEL and vol% range which can make accurate blend measurements accounting for variability in the blend and changing LEL An ability to measure the concentration of H2 and adjust calibrations accordingly, to allow accurate use across both NG and blended networks
Whats needed going forward? .. continued The ATEX gas group for a 20 vol% H2 blend with NG remains as IIA as per BS EN 60079-20-1:2010 Up to 25 vol% blend remains IIA Pure H2 has an ATEX gas group of IIC Approval for use in the gas industry may differ, but for Cadent: Gas detectors must be compliant with BS EN 60079-29-1: Explosive Atmospheres, Gas detectors, Performance Requirements Of Detectors For Flammable Gases IGEM /SR/29 dealing with gas escapes contains: