ATMPs Quality Control and Logistics Strategy
This information focuses on the quality control, shelf-life, and logistics aspects of Advanced Therapy Medicinal Products (ATMPs). It delves into key characteristics of ATMPs, methods for analyzing and evaluating them, storage protocols, transportation considerations, and real-time monitoring approaches. It stresses the importance of maintaining critical quality attributes during logistics processes and emphasizes the need for designing quality into logistics strategies from the outset. Several projects and strategies related to establishing QC and logistics for cell-based products are highlighted, along with the significance of suitable methods for QC testing and transportation validation in clinical trials. The content also addresses the qualification process for shipping containers, ensuring they are fit for purpose.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
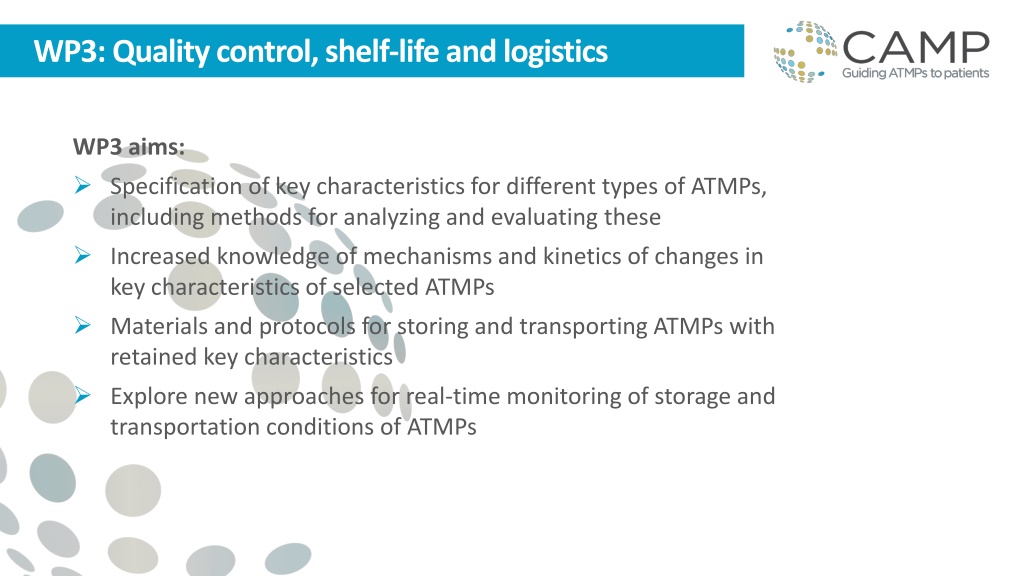
WP3: Qualitycontrol, shelf-lifeand logistics WP3 aims: Specification of key characteristics for different types of ATMPs, including methods for analyzing and evaluating these Increased knowledge of mechanisms and kinetics of changes in key characteristics of selected ATMPs Materials and protocols for storing and transporting ATMPs with retained key characteristics Explore new approaches for real-time monitoring of storage and transportation conditions of ATMPs
Impact of logistics on manufacturing strategy Critical quality attributes should not be altered during shipping, freezing, thawing or storing Fresh If therapies do not allow freezing/thawing Multiple manufaturing site Need to contol regulatory, manufacturing equivalence, etc. Cryo Single large scale manufacturing site possible Reduced cell viability How to manage/control thaw
Think about strategy during development Key to logistics success is designing in quality from the outset Clinical development Manufacturing development Logistical development
WP 3 2018 2019 2020 2121 2022 2023 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 3.1 - Establishing a QC and logistics strategy of cell-based products for secure patient treatment Jim Lund 3.4 Generic logistics project with multiple stakeholders 3.2 - Single cell qPCR characterization of ATMP Eva Bom 3.3 Establishing a dryice based logistics strategy for cell therapies Jim Lund 48 72 60 36 12 24 Ver200121
3.1 Establishing a QC and logistics strategy for autologous cell sheet Establish suitable methods for QC testing Identifying and validating the transportation for a proposed clinical trial
The shipping container must be fit for purpose Qualification is an inspection and testing process used to establish that a piece of equipment or a physical installation is fit for purpose in the operational context within which it will be used. There are typically 3 stages in the process: Design or installation qualification (DQ) Test of the container itself Operational qualification (OQ) Ambient temperature testing in lab Performance qualification (PQ) Conducted as a field test Must be successfully performed 3 times 6 Ref: WHO technical supplement to technical report series, No. 961, Suppliment 12 and 13, 2015
3.1 Establishing a QC and logistics strategy for autologous cell sheet Establish suitable methods for QC testing Identifying and validating the transportation for a proposed clinical trial 7
3.1 Establishing a QC and logistics strategy for autologous cell sheet Shipment of a product @~37 C with 48h shelf-life within EU is feasible 9
 undefined
undefined


 undefined
undefined