Understanding Institutional Review Board (IRB) Process
Institutional Review Board (IRB) plays a crucial role in ensuring research involving human subjects adheres to ethical standards. This summary provides insights into the IRB process, including determining the need for IRB review, defining human subjects research, and the importance of human subjects protections training for study personnel.
- IRB Process
- Human Subjects Research
- Ethical Standards
- Research Ethics Training
- Institutional Review Board
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
IRB Process Updated December 6, 2019
Institutional Review Board What is an IRB? What is its job? What are its guiding Principles? Belmont Report (i.e., Respect for Persons, Beneficence, Justice) Code of Federal Regulations, Title 45 Public Welfare, Part 46 Protection of Human Participants (aka The Common Rule) USM Policies and Procedures, Section III and Section IV
IRB Process Step 1 Determine Whether Your Study Requires IRB Review
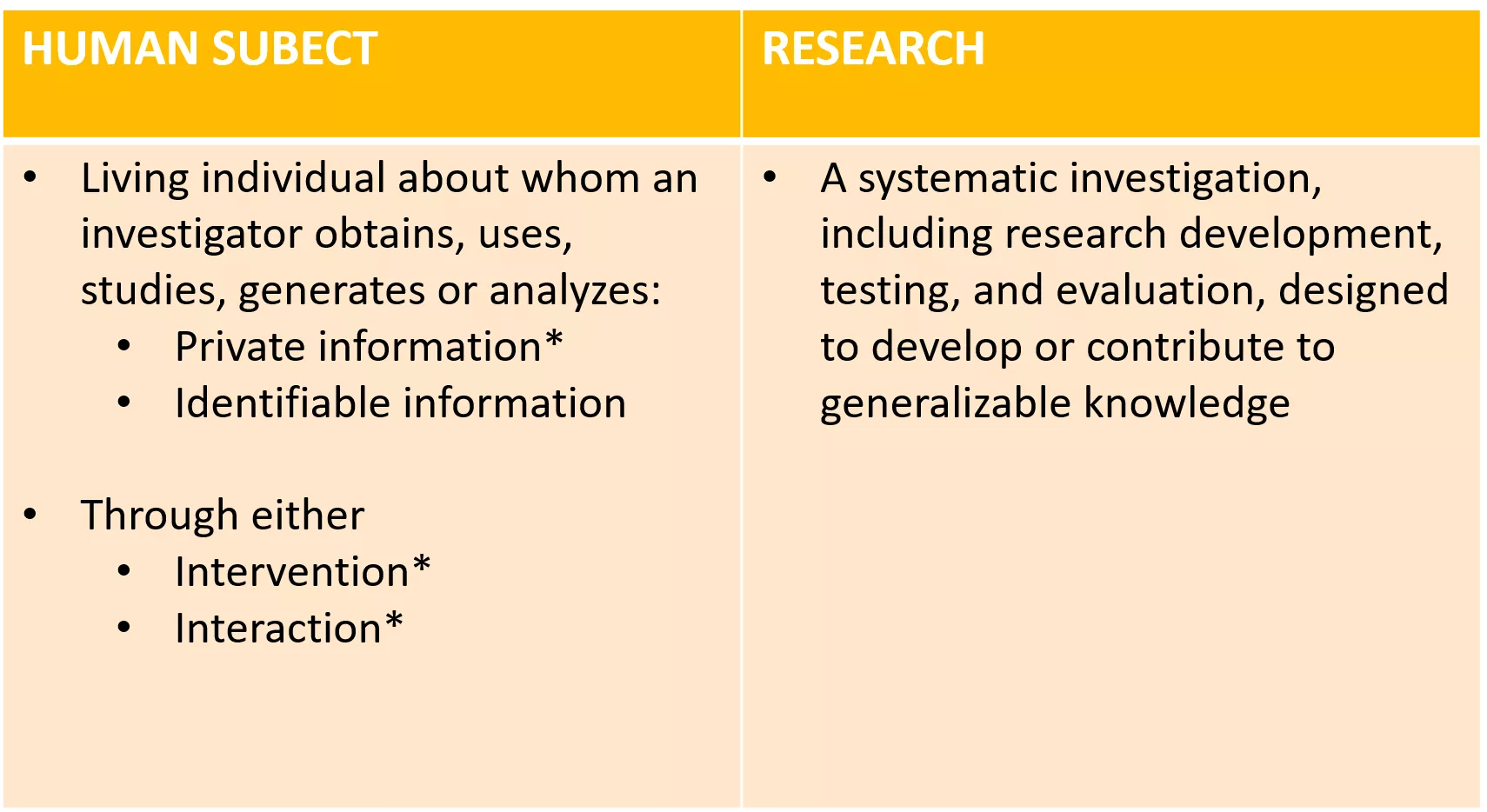
Determine if your study is human subjects research What is Human Subjects Research? HUMAN SUBECT RESEARCH Living individual about whom an investigator obtains, uses, studies, generates or analyzes: Private information* Identifiable information A systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge Through either Intervention* Interaction*
IRB Process Step 2 Ensure All Study Personnel Have Completed Human Subjects Protections Training
Human Subjects Protections Training CITI PROGRAM Login through CITI with your TU netID and password. Complete all modules Should take several hours Score at least 80% The CITI website is already linked to Towson s Kuali Protocols, so when you submit your IRB application online your certificate (as well as other TU personnel listed on your protocol) will automatically connect to your application. Non-TU personnel will need to have their certificates attached separately in the Attachments section. If you have completed another NIH approved human subjects training program through another institution that is still active and up-to-date, TU will accept that in lieu of a TU CITI certificate. NOTE: Studies will not be submitted for review UNTIL evidence of Human Subjects Protection Training has been provided by ALL study personnel involved in data collection or analysis
Maintaining Training Certificates If you have research assistants (RAs) working on a protocol, those certificates do not have to be on file with the IRB since RAs frequently change from semester to semester for course requirements. It is the PI s responsibility to ensure their students complete the appropriate training and have them on file. Failure to do so may result in suspending a protocol until all trainings have been completed.
IRB Process Step 3 Determine Level of Review
Determine if your study meets the definition of minimal risk As defined in 45CFR46.102.j, minimal risk . . . means that the probability and magnitude of harm or discomfort, anticipated in the research are not greater in and of themselves than those risks ordinarily encountered in daily life or during the performance of routine physical or psychological tests.
TYPES OF RESEARCH AND REVIEW CATEGORIES Types of Research Exempt Expedited Full Board Minimal risk research Data are anonymous OR No sensitive information collected Meets specific criteria Minimal risk research Sensitive information is collected Reasonable protections are in place to ensure confidentiality Meets specific criteria Greater than minimal risk Does not meet criteria for Exempt or Expedited research TU Review Categories Accelerated Review Standard Review Full Board Review Continuing Review? Annually via Standard Review, Unless * Progress Report every 3 years No Unless * (a) you intend to modify the study procedures or consent document; (b) a participant complains about the research or there is an adverse event; (c) there is a protocol violation/deviation; and/or (d) new findings indicate that the study risks, or the risk/benefit ratio, have change
Review Categories Accelerated Review Standard Review Full Board Review 3rd Friday of each Month 3rd Friday of each Month Deadline Rolling Form Request for Accelerated Review - Choose either Adult or Minor/Legal Minor version Request for Standard or Full Board Review Request for Standard or Full Board Review Reviewer Chair, Assistant Chair, or Single IRB Member Single IRB Member Quorum of the IRB Time until Feedback or Approval ~ 2 weeks ~ 3 weeks ~ 4 weeks
IRB Process Step 4 Complete and Submit the Application
The Successful IRB Application Provides detail about: Study rationale as well as aims or hypotheses Anticipated benefits Inclusion/Exclusion criteria Recruitment plan How informed consent will be obtained* Provides detail about: Study procedures (i.e., in chronological order, what will subjects be asked to do)* Measures to be used Anticipated risks and plans for mitigating or managing risks If applicable, a statement that there are no foreseeable risks
Continued Provides detail about: How confidentiality or anonymity will be assured How data will be stored to ensure security and who has access to data If/when/how data will be destroyed* If/how subjects will be compensated Informed Consent/Assent** Includes all required elements (use TU Consent/Assent template) 8th grade reading level (adults) Appropriate reading level (children/adolescents) Sufficient detail so that participants understand risks and what is expected of them ** If data are anonymous, use an information sheet
Continued At minimum, attach the following: Informed consent form or information sheet Measures (or detailed description of questions, if proprietary), interview questions Human Subjects Protections Training certification for external investigators, or those who have not completed training through TU s CITI webpage If student PI, Faculty Advisor Agreement If appropriate also attach: Informed assent form Recruitment materials, including: Content of e-mails or letters Scripts for verbal recruitment Fliers Content of ads and where they will be placed Links to relevant websites
IRB Process Step 5 Revising Your Application Based on Reviewer Comments
Revising your Application Address ALL reviewer concerns Submit a cover letter with the reviewer s comments and your response/revision If you disagree with a suggested revision In your response, provide a detailed explanation for why you disagree with the comment; be advised the reviewer may still require the revision if the rationale is not convincing Submit a revised protocol AND consent form Use track changes, highlighting, or different colored font for revisions. Kuali Protocols has a feature that allows you to do this when revising the online form. However, you MUST use these features in Word or PDF documents that are attached separately as well. DO NOT BEGIN DATA COLLECTION UNTIL APPROVAL FOR THE STUDY HAS BEEN GRANTED!! Once approved, you will receive notification from the IRB office you may begin data collection!
IRB Process Step 6 Amending Your Application After Approval
Amending Applications All substantive changes to consent forms or protocols MUST be reviewed and approved by the IRB If not sure if change is substantive, just ask! Submit Open your protocol on Kuali Protocols and click on Amend in the right hand column of actions to choose from Each section of the online form will now have an option to edit. These edits will have track changes applied to them for the IRB. To view them as track changes click on the Show Latest Changes box at the top of the form. Make sure to complete the Justification box at the top for a clear rationale on why you are amending the protocol. Include any other revised attachments with track changes, highlighting, or different colored font. Include: new questionnaires; recruitment materials, etc Click Submit in the right hand column of actions to choose from. NOTE: You may not implement protocol changes until the IRB has approved the amendment
Some Final Thoughts What to do if . . . I want to include 17-year-old college students I want to pay participants I want to use data panels Someone complains about the research A participant experiences an adverse event There is a protocol deviation/violation NOTE: For more detailed information on all of these topics, see the Principal Investigator s Instruction Manual which is available on the IRB website