Understanding the Particle Theory of Matter
Matter, as described by the Particle Theory, is composed of tiny particles in constant motion with spaces between them. This theory, formulated by John Dalton, explains the behavior of matter based on four principles. It elaborates on the structure, motion, and interactions of particles within substances, illustrating how matter exists in various states and forms.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
John Dalton the guy who came up with particle theory
Agenda What is matter The Particle Theory Mixtures vs. Pure Substances Mixtures Hetrogeneous Mixtures Solution Homogeneous Mixtures Mechanical Mixture Suspension Solution PureSubstances Elements Compounds
What is Matter? Matter is anything that contains mass & volume (takes up space) Energy, such as light, heat, and sound, is NOT matter IT S ALL MATTER!
The Particle Theory of Matter This provides a way to describe the structure and behaviour of matter. There are 4 principals of the particle theory written by a chemist named John Dalton. Think About it! Look at a piece of chalk If you broke a piece of chalk in half, would it still look like and behave like chalk? How small of pieces would you have to break a piece of chalk so that it isn t chalk anymore is that even possible? The answer is YES, because the smallest pieces of chalk are made up of particles and the particle theory explains how this works!
The Particle Theory of Matter 1. All matter is made up of very tiny objects called particles. These particles are VERY tiny too small to be seen with any regular light microscope
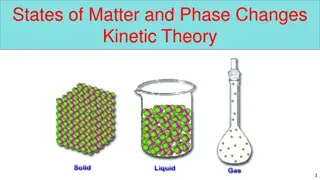
The Particle Theory of Matter 2. All Particles have spaces between them. The size of these spaces determine the state of the matter
The Particle Theory of Matter 3. Particles present in matter are always in motion. In a solid, they vibrate together In a liquid, they stay close together but slide along each other In a gas, they bounce and move in all directions!
The Particle Theory of Matter 4. The particles in a substance attract each other. The degree that particles are attracted to each other is different in difference substances, Take a guess! Which substance do you think has the strongest attractions? Which has the weakest? Iron Water Oxygen
The Particle Theory of Matter Recap: Here they are all again All matter is made up of very tiny objects called particles. 1. 2. All particles have spaces between them. 3. Particles present in matter are always in motion. 4. The particles in a substance attract each other.
Pure Substances vs. Mixtures All matter can be divided into two big categories: PURE SUBSTANCES and MIXTURES. Pure substances are made up of one type of element or compound Mixtures are a combination of pure substances (2 or more types of particles) Versus
Pure Substances Pure substances are made up of only ONE type of particle Pure substances are in the form of either elements or compounds.
Elements Elements are the smallest and purest forms of particles They cannot be broken down further by ordinary means (such as simple reactions, heat, or electricity) They are only made up of one type of atom (one type of particle) Examples are Oxygen, Calcium, Iron, Carbon and Helium
Compounds A compound is made up of only one type of particle, called a molecule Compounds are made up of many molecules that are held together by chemical bonds! Water is a molecule because it is made out of two types of atoms (oxygen and hydrogen) Hydrogen Oxygen Water is a compound because it has many molecules of one type (H2O = Water) Hydrogen
Mixtures A mixture is a combination of two or more different substances (different types of particles). Mixtures can be divided into 2 big categories: Heterogeneous Mixtures Homogeneous Mixtures
Heterogeneous Mixtures Made up of two or more particles where the different particles are easy to see and separate. Can be divided into two categories: Mechanical mixtures Suspensions An Oreo cookie is a heterogeneous mixture
Mechanical Mixtures Particles that are in the same container but can be easily separated. Made up of several distinct parts A lasagne, parfait, and a cookie are both examples of mechanical mixtures THINK ABOUT IT! What are some of the parts of these mechanical mixtures?
Suspensions Small particles of one substance float in another substance Salad Dressing, glittery nail polish, and yogurt are examples of suspensions
Homogeneous Mixtures A homogenous mixture occurs when 2 or more particles blend together and cannot be seen separately Solutions are homogeneous mixtures!
Solutions A mixture of 2 or more things where one substance dissolves Kool-Aid and tea are solutions because you cannot see the flour crystals floating in the Kool-Aid, and you cannot see the tea floating in the tea when the bag is removed Kool-Aid and tea are examples of solutions