Understanding States of Matter: Kinetic Molecular Theory and Phase Transitions
Exploring the fundamental concepts of the states of matter, this content delves into the Kinetic Molecular Theory, explaining how matter changes from solid to liquid to gas based on particle movement and energy levels. It further discusses the nature of gases and liquids, vaporization, evaporation, and the cooling process involved in evaporation. Through images and explanations, it provides a comprehensive overview of these key principles in chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
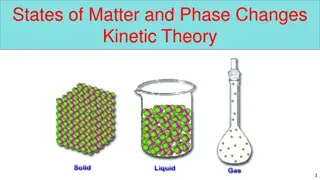
States of Matter 19-23 odd
Kinetic Molecular Theory Explains how matter changes from Solid (s) Liquid (l) Gas (g) There are spaces between particles of matter, and these particles are consistently moving. All particles have energy. The amount of kinetic energy depends on whether it is a s,l,g. Solids least amount of kinetic energy Gas- greatest amount of kinetic energy Remember kinetic = motion
Continued Temperature of a substance is a measure of the average kinetic energy. A drink at 25 C Majority of molecules at 25 C Some above and some below 25 C
Nature of Gases Gas Pressure result of collisions of many rapidly moving particles with walls of container (pascals, mmHg, atm) Atmospheric Pressure- force of the weight from the air above. http://apphysicsc.com/wp-content/uploads/2012/08/MercuryBarometer.jpg
Nature of Liquids Key difference between gasses and liquids: Gasses attractive forces (no defined volume) Liquids = attractive forces (defined volume since atoms stay close together)
Vaporization/Evap/Boil Vaporization of an element/compound is a phase transition from the liquid phase to gas phase. Evaporation (below boiling) Boiling (above boiling)
Evaporation Evaporation is a phase transition from the liquid phase to gas phase that occurs at temperatures below the boiling temperature at a given pressure. This process does not involve the addition of heat (kinetic energy) Examples of evaporation: Ice cubes in freezer, pool water, sweat
Evaporation continued When molecules collide they transfer energy to each other in varying degrees (based on how they collide). Transfer of energy can be one sided Molecules that receive enough energy can escape as vapor. These molecules carry kinetic energy (heat) and therefore makes evaporation a cooling process (ex. Sweat)
Boiling (Vapor Pressure) Boiling is a phase transition from the liquid phase to gas phase that occurs at or above the boiling temperature Need to understand vapor pressure first: Vapor Pressure: measure of the force exerted by a gas above a liquid
Boiling cont Boiling Point occurs when vapor pressure (pressure from escaping atoms) is equal to atmospheric pressure. Standard boiling points of substances are expressed at 1 atm. Ammonia -28.1 F Jet Fuel 350 F Olive Oil 570 F http://www.pumpworld.com/images/VaporPressure.gif
Changing Boiling Points You travel up a mountain to a higher elevation. It becomes harder to breathe because the amount of air molecules is less. You also notice your ears are popping due to the lower atmospheric pressure. You decide to make some hot chocolate. Will the cup of water boil at a higher or lower temperature here?
Lower! The water molecules have less atmospheric pressure that needs to be overcome by vapor pressure. Remember boiling when vapor pressure meets/exceeds atmospheric pressure. 32 F(Freeze) 212 F (Boil) http://www.pumpworld.com/images/VaporPressure.gif
Nature of Solids Fixed volume and shape How do solids melt? When heat is applied, particles vibrate rapidly due to the increase in kinetic energy. Meting Point: point where solid changes to a liquid. Note: the melting point and freezing point of a substance are equal.
http://blogs.agu.org/georneys/files/2011/09/Crystal_Lattice.gifhttp://blogs.agu.org/georneys/files/2011/09/Crystal_Lattice.gif Crystals Solids form crystals Crystals -particles arranged in an orderly repeating, 3D pattern. http://t0.gstatic.com/images?q=tbn:ANd9GcQGg6cbIFTEMmlCKOvW1ozg8ozG02uBAanBbSqc5HgHk5yrB5Is http://www.satyacenter.com/item_files/article_574_8.jpg http://www.chemistryland.com/CHM107/EarlyChemistry/StoneTools/CubicStructureNaCl.jpg
Amorphous Solids Not all solids form crystal structures Amorphous solids lack ordered internal structure (rubber, plastic, asphalt, glass).
Allotropes Two or more different molecular forms of the same element in the same physical state: Carbon (diamond and graphite)