Understanding Surface Chemistry and Adsorption Processes
Surface chemistry is the study of processes at the interface of two bulk phases, such as liquid-liquid interactions. Adsorption and absorption are key phenomena where atoms, ions, or molecules adhere to surfaces or enter bulk phases. The accumulation of molecular species at the surface, known as adsorption, has various practical applications like gas purification and dye removal. This content explores the differences between adsorption and absorption, highlighting examples and the action of adsorption in various scenarios.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
INTRODUCTION Surface chemistry is the study of processes that occur at the interface of two bulk phases. The bulk phases can be of the type: Liquid -liquid
TYPES ADSORPTION: is the adhesion of atoms, ions, biomolecules or molecules of dissolvedsolidsto asurface. gas, liquid, or ABSORPTION: is a physical or phenomenon or a process in which atoms, molecules, or ions enter some bulk phase - gas,liquid, or solidmaterial. chemical
Adsorption onactivated charcoal.
Absorption through a membrane
Adsorption Adsorbate Adsorbent Absorption( partitioning ) PHASEI PHASE2
Adsorption Theaccumulation of molecular speciesat the surfacerather than in the bulk of asolid or liquid istermed adsorption . The molecular species or substance, which concentrates or accumulatesat the surfaceistermed adsorbateandthe material on the surface of which the adsorption takes place is called adsorbent.
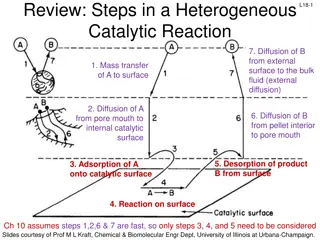
Action ofadsorption (i) If a gas like O2, H2, CO, Cl2, NH3 or SO2is taken in a closed vessel containing powdered charcoal,it isobservedthat the pressure of the gasin the enclosedvessel decreases. The gas molecules concentrate at the surface of the charcoal, i.e., gases areadsorbedat the surface. (ii) In a solution of an organic dye, say methylene blue, when animal charcoal is addedandthe solution iswell shaken,it isobservedthat the filtrate turns colourless. The molecules of the dye, thus, accumulate on the surface of charcoal, i.e., are adsorbed. (iii) Aqueous solution of raw sugar,when passedover bedsof animalcharcoal, becomescolourless asthe colouringsubstancesareadsorbedbythe charcoal. (iv) Theair becomesdry in the presenceof silicagel becausethe water molecules get adsorbedon the surfaceof the gel. Theprocessof removing anadsorbedsubstancefrom asurfaceon which it is adsorbed is calleddesorption.
Difference between adsoprtion andabsorption In adsorption, the substanceisconcentrated only at the surfaceand doesnot penetrate through the surfaceto the bulk of the adsorbent, while in absorption the substance is uniformly distributed throughout the bulk of the solid. A distinction can be made between absorption and adsorption by takinganexample of water vapour.Watervapoursareabsorbedby anhydrous calciumchloridebut adsorbed bysilicagel. In other words, in adsorption the concentration of the adsorbate increasesonly at the surfaceof the adsorbent, while in absorption the concentrationisuniform throughout the bulk of the solid. Both adsorption andabsorptioncantake placesimultaneouslyalso. Theterm sorption isusedto describeboth the processes.
Mechanism of adsoprtion Inside the adsorbent all the forces acting between the particles are mutually balanced but on the surface the particles are not surrounded by atoms or molecules of their kind on all sides, and hence they possess unbalanced or residual attractive forces. These forces of the adsorbent are responsible for attracting the adsorbate particles on its surface.Theextent of adsorption increaseswith the increaseof surfaceareaper unit massof the adsorbent at agiventemperatureandpressure.
Another important factor featuring adsorption isthe heat of adsorption. During adsorption, there is always a decrease in residual forces of the surface,i.e.,thereisdecreasein surfaceenergywhich appearsasheat. Adsorption, therefore, is invariably an exothermic process. In other words, H of adsorption is always negative. When a gas is adsorbed, the freedom of movement of its molecules become restricted. Thisamounts to decrease in the entropyof the gasafter adsorption,i.e., Sisnegative. Adsorption isthus accompaniedby decreasein enthalpy aswell asdecrease in entropy of the system. For a process to be spontaneous, the thermodynamic requirementisthat, at constant temperatureandpressure, Gmustbenegative,i.e.,there isadecreasein Gibbsenergy. On the basis of equation, G = H T S, G can be negative if H has sufficiently high negative value as T Sispositive. Thus,in anadsorption process,which isspontaneous, acombination of these two factors makes G negative.As the adsorption proceeds, H becomes less and less negative ultimately H becomes equal to T S and G becomes zero. At this stateequilibrium is attained.
Types ofadsorption There are mainly two types of adsorption of gases on solids. If accumulationof gason the surfaceof asolid occurson accountof weak vanderWaals forces,the adsorptionistermedasphysicaladsorptionor physisorption. Whenthe gasmolecules or atomsareheld to the solidsurfacebychemical bonds,the adsorptionistermedchemicaladsorptionorchemisorption. The chemical bonds may be covalent or ionic in nature. Chemisorption involvesahigh energyof activationandis,therefore, often referredto as activated adsorption. Sometimes these two processes occur simultaneously and it is not easyto ascertain the type of adsorption. Aphysical adsorption at low temperature maypassinto chemisorption asthe temperature isincreased. Forexample, dihydrogenisfirst adsorbedon nickelbyvanderWaals forces. Molecules of hydrogen then dissociate to form hydrogen atoms which are heldon the surfacebychemisorption.
Characteristics ofphysisorption (i) Lackofspecificity: Agivensurface of anadsorbent doesnot showanypreference for a particulargasasthe vander Waals forces areuniversal. (ii) Natureofadsorbate:Theamount of gasadsorbedbya soliddependson the nature of gas.In general,easilyliquefiablegases(i.e., with higher criticaltemperatures)are readilyadsorbedasvander Waals forces arestronger nearthe criticaltemperatures. (iii) Reversible nature:Physical adsorption of a gas by a solid is generally reversible. Thus, More of gas is adsorbed when pressure is increased as the volume of the gas decreasesandthe gascanberemoved bydecreasing pressure.Sincethe adsorption processisexothermic,the physical adsorption occursreadily at low temperatureand decreaseswith increasingtemperature(Le-Chatelier sprinciple). (iv) Surface area of adsorbent:The extent of adsorption increases with the increase of surface areaof the adsorbent.Thus,finely dividedmetalsandporous substanceshaving largesurfaceareasaregood adsorbents. (v) Enthalpyofadsorption:Nodoubt, physical adsorption isanexothermic processbut its enthalpy of adsorption isquite low (20 40kJmol-1).Thisisbecausethe attraction between gasmoleculesandsolid surface isonlydueto weakvanderWaals forces.
Characteristicsof chemisorption (i)Highspecificity:Chemisorption ishighlyspecific andit will only occur if there is some possibility of chemical bonding between adsorbent andadsorbate. (ii) Irreversibility:As chemisorption involves compound formation, it isusuallyirreversibleinnature. -Chemisorption is also an exothermic process but the process is very slow at low temperatures on accountof highenergy of activation. Like most chemical changes, adsorption often increases with rise of temperature. -Physisorption of agasadsorbed at low temperature maychangeinto chemisorption at a high temperature. Usually high pressure is also favourable forchemisorption.
(iii) Surfacearea:Likephysicaladsorption, chemisorption also increaseswith increaseof surfaceareaof the adsorbent. (iv) Enthalpyof adsorption:Enthalpy of chemisorption ishigh (80-240kJmol-1)asit involves chemicalbond formation.
Adsorptionisotherms Thevariation in the amount of gasadsorbed by the adsorbent with pressure at constant temperature can be expressed by meansof acurve termed asadsorption isotherm. Freundlichadsorption isotherm: Freundlich,in 1909,gavean empirical relationship between the quantity of gasadsorbed by unit mass of solid adsorbent and pressure at a particular temperature. The relationship can be expressed by the following equation:
where x is the mass of the gas adsorbed on mass m of the adsorbent at pressure P, k and n areconstantswhich dependon the nature of the adsorbent and the gas at a particulartemperature. The relationship is generally representedin the form of acurve where mass of the gas adsorbed per gram of the adsorbent is plotted against pressure. These curves indicate that at a fixed pressure, thereis a decrease in physical adsorption with increase in temperature. These curves always seem to approach saturation at highpressure.
Takinglogon both sidesof equation we get Thevalidityof Freundlichisothermcanbe verified by plotting log x/m on y-axis (ordinate) andlogp on x-axis(abscissa). If it comes to be a straight line, the Freundlich isotherm is valid, otherwise not .Theslopeof the straight linegives the valueof 1/n.Theintercept on the y- axisgivesthe valueof logk. Freundlich isotherm explains the behaviour of adsorption in an approximate manner. The factor 1 can have values between 0 and 1 . Thus, aboveequation holdsgood overalimited range ofpressure.
When1/n=0,x/m=constant,the adsorption isindependent of pressure.nm When1/n=1,x/m=k p, i.e.x p, the adsorptionvariesdirectly with pressure. Both the conditions aresupported by experimental results. The experimental isotherms always seem to approach saturation at highpressure. Thiscannot beexplained by Freundlich isotherm.Thus,it fails at highpressure.
Adsorption fromsolution phase Solids can adsorb solutes from solutions also. When a solution of acetic acid in acetic acid is shaken with charcoal a part of it is adsorbed by charcoalandthe concentrationof the aciddecreasesin the solution. .The following observations have been made in the case of adsorption from solution phase: (i) Theextent of adsorptiondecreaseswith anincreasein temperature. (ii) Theextent of adsorptionincreaseswith anincreaseof surfaceareaof the adsorbent. (iii) Theextent of adsorptiondependson the concentrationof the solute in solution. (iv) Theextent of adsorptiondependson the natureof the adsorbentand the adsorbate.
Freundlichs equation approximately describes the behaviour of adsorptionfrom solution with adifferencethat insteadof pressure, concentrationof the solution istakeninto account,i.e. WhereCisthe equilibrium concentration,i.e.,when adsorptionis complete.Ontakinglogarithmof the aboveequation,we have Plotting log x/magainstlog Castraight line isobtainedwhich showsthe validity of Freundlichisotherm. This can be tested experimentally by taking solutions of different concentrations of aceticacid.Equalvolumesof solutions areaddedto equalamountsof charcoalin differentflasks. The final concentration is determined in each flask after adsorption. The differencein the initial andfinal concentrationsgivethe valueof x.Using the aboveequation,validity of Freundlich isothermcanbeestablished.
Applications ofadsorption (i) Production of high vacuum: Theremaining traces of air can beadsorbedby charcoalfrom avesselevacuatedby avacuum pump to give averyhigh vacuum. (ii) Gas masks:Gas mask (a device which consists ofactivated charcoal or mixture of adsorbents) is usually used for breathing in coalmines to adsorb poisonous gases. (iii) Control of humidity: Silicaandaluminium gelsareusedas adsorbents for removing moisture andcontrolling humidity.
(iv) Removalof colouring matter from solutions:Animalcharcoalremoves coloursof solutions byadsorbingcolouredimpurities. (v) Heterogeneouscatalysis:Adsorptionof reactants on the solidsurfaceof the catalysts increases the rate of reaction. There are many gaseous reactionsof industrial importanceinvolving solidcatalysts. (vi) Separation of inert gases:Dueto the differencein degreeof adsorption of gases by charcoal, a mixture of noble gases can be separated by adsorptionon coconutcharcoalat different temperatures. (vii) In curing diseases:Anumberof drugsareusedto kill germsbygetting adsorbed onthem. (viii) Froth floatation process: Alow grade sulphide ore is concentrated by separating it from silica and other earthy matter by this method using pine oil and frothingagent . (ix) Adsorption indicators: Surfaces of certain precipitates such as silver halideshavethe property of adsorbing somedyeslike eosin, fluorescein, etc.andtherebyproducingacharacteristiccolour at the endpoint.
(x) Chromatographic analysis: Chromatographicanalysis based on the phenomenon of adsorption finds a number of applicationsin analytical andindustrial fields.
Electrophoresis When electric potential is applied across two platinum electrodes dipping in a colloidal solution, the colloidal particles movetowardsoneor the other electrode. The movement of colloidal particles under an applied electric potential is called electrophoresis. Positively charged particles movetowardsthe cathode while negatively charged particles move towards theanode When electrophoresis that is.,movement of particles ispreventedbysomesuitable means, it is observed that the dispersion medium begins to move in an electric field. This phenomenon is termed electroosmosis.