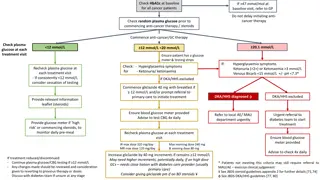
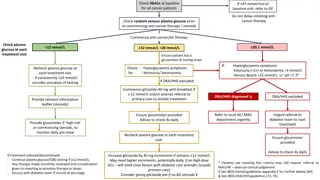
Understanding Insulin Pump Therapy and Continuous Glucose Monitoring
Exploring the basics of insulin pump therapy, including the basal/bolus concept, operation of insulin pumps, advantages and limitations, day-to-day living with a pump, continuous glucose monitoring, and available systems.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Mindy Saenz RDN, LDN, CDE I Wear My Pancreas on the Outside: Insulin Pump and CGM Basics Clinical Dietitian/Certified Diabetes Educator Brody School of Medicine at East Carolina University. Pediatric Specialty Care Greenville, NC
Objectives Review basal/bolus concept Describe how insulin pumps work Discuss advantages and disadvantages of insulin pump therapy Discuss day to day living with a pump Discuss how continuous glucose monitoring works Describe benefits and limitations of continuous glucose monitoring Describe hybrid closed loops systems Describe the currently available insulin pump and continuous monitoring systems.
Basal/bolus concept Mixing of short and intermediate insulin led to thinking of insulin action in blocks of time i.e. AM NPH covers lunch and afternoon, PM Regular covers time after dinner, etc. One part of the insulin acts as a basal component, the other as a bolus Insulin analogs permit thinking of the insulin based on it s action. Long lasting or basal insulins are relatively flat and last 24 hours or longer and provide insulin overnight and between meals. Rapid acting or bolus insulins give coverage for meals and for correcting high blood glucose levels. Pumps use only rapid acting insulin for both basal and bolus requirements.
BID NPH and Regular (Or 70/30 bid) _______________________ B L S HS
4 Shots with Basal and Rapid-Acting Insulin Rapid-acting Rapid-acting Rapid-acting Basal ________________________ B L S HS
Insulin pump ________________________ B L S HS
Insulin pumps All pumps have the same basic components and deliver a pre-programmed basal rate and then are programmed to give bolus insulin on demand for food or high blood glucose levels The differences are the additional features such as no tubing, color screen, built in CHO database, ability to bolus from the meter, integrated CGM, waterproof, etc. Components of insulin pumps: Reservoir to hold 3 days worth of insulin Motor to push the insulin into the patient Menu-driven operating system Battery and casing All but OmniPod also have an infusion set (tubing) to get the insulin from the reservoir to the patient.
Advantages of pumps Multiple basal rates Temporary basal rates Multiple basal patterns Avoidance of long/intermediate-acting insulin More precision in insulin delivery More accurate dosing for food intake-fractions of a unit Bolus calculators Easier correction of high BGs Weight management Improved glucose levels and reduced A1c Lifestyle flexibility-shift work, travel, etc. Fewer injections/needles You have the tools you need on your body all the time Decreased hypoglycemia Less anxiety trying to keep on schedule Increased control with exercise Child safety features Improved quality of life
Disadvantages of pumps Risk of DKA if insulin delivery is interrupted Being attached-constant reminder of diabetes Privacy-visible in public and may promote unwanted questions. Skin issues Infusion set issues Missing boluses Weight gain Machines can fail and break Cost
Pump therapy indications Inadequate BG control Frequent hypoglycemia Dawn phenomenon Exercise Pediatric Pre-conception/pregnancy Hectic lifestyle Shift work Gastroparesis ? Anyone with diabetes
Patient Selection Criteria Motivated to take insulin Wants to improve glucose control Desires to live a more normal life Has family support Ability to problem solve Has realistic expectations Willing to monitor and record BG-minimum of 4 per day (can be less if wearing CGM) Willing to quantify food intake Willing to follow-up
Which pump? Provide the names of the companies and their brochures to the patient Explain the similarities and differences Let the patient choose!
Living with a pump-where to wear Clipped to belt In a pocket Tucked in bra Special pump clothing with pockets sewn inside Pump accessories such as a thigh thing , zipper bag or back harness are sold by the companies
Living with a pump-sleep Clip to pajamas or underwear Put it under a pillow Buy special pump clothing with pockets Leave it loose next to you
Living with a pump-bathing Most pumps are watertight, but usually patients simply disconnect and leave it on the counter
Living with a pump-intimacy Can disconnect have to make sure to reconnect prior to falling asleep Leave it connected and just move it if it gets in the way
Living with a pump-exercise/sports Most people just wear it and use a lower temporary basal rate For intense activity they can disconnect-check BG every hour and reconnect and take a small bolus if needed Most kids choose to disconnect and may even need extra food in addition to disconnecting.
Continuous Glucose Monitoring (CGM)
CGM changes the way we interpret glucose data Self-monitoring of BG provides only a snapshot - and many patients don t reach targets CGM provides Real time information about current glucose concentration Feedback about the effectiveness of intervention Warnings when glucose concentrations become dangerously high or low (except Libre) Alerts can be used preemptively to avoid hypo-and hyperglycemia
CGM components Sensor-thin flexible wire inserted beneath the skin into the interstitial fluid space using an introducer needle (26 gauge to 22 gauge) Transmitter- seated into a plastic pod or clicked into the side of the sensor, Libre is all one piece Receiver- either pump, phone or separate
CGM systems Display and record glucose levels throughout the day and night (Libre only when scanned) Provide direction and rate of change of glucose information to help maintain euglycemia Provide alerts if glucose is traveling outside of targets (not Libre) Measure glucose in the Interstitial Fluid space Use an electrochemical process for sensing glucose Currently 4 companies with FDA approved patient systems.
CGM Benefits Limitations Alarms Annoying and disruptive Cannot always be heard Expensive Time consuming Must be worn frequently to get benefits Alerts for lows & highs (not on Libre) Rate of change alarms help prevent lows and highs Identify trends for dose adjustments Immediate feedback on how changes in diet, exercise and insulin affect BG Decreased variability-less hypo-/ hyperglycemia ***Patients/Parents need to have reasonable expectations
People choose cgm for: Better glucose management Avoiding frequent hypoglycemia Preventing hypoglycemia unawareness Understanding the effects of particular foods on post meal BG Overnight safety Living alone Frequent driving, travel, high-risk professions Young children unable to report hypoglycemia Tight control before and during pregnancy Avoiding complications
Who should use CGM Person and family are interested in it Person is willing to wear a sensor Stable and adequate diabetes care team to help manage the info Willingness to change the way they think about BG and insulin dosing Enough body fat to wear the sensor Able to afford it
Artificial Pancreas (Closed Loop System/hybrid closed loop)
Hybrid closed loop The currently FDA approved system and those looking to be approved over the next year are hybrid closed loop systems. This means that they can deliver insulin based on sensor readings using an algorithm to either increase or decrease the persons insulin as long as certain conditions are met. They all require input when the person eats carbohydrate. They still require site and sensor changes and fingersticks may still be required for calibration and algorithm updates. Require more training and work on the part of the patient and the healthcare team. Patients and families need to have realistic expectations.
Omnipod DASH Mobile Apps Omnipod VIEW App Omnipod DISPLAY App Today View Widget 12 Caregivers can view Pod info on personal Smartphone CGM and Pod single screen view on iOS devices Secondary PDM display on personal Smartphone The Dexcom System does not have integrated functionality with the Omnipod DASH System
Medtronic Minimed 630g with enlite
Medtronic Minimed 670g with guardian
What we need for fully closed loop? Faster rapid-acting insulin Better sensors with improved accuracy and less lag time Consideration of the use of other hormones (glucagon, amlyin, leptin) Algorithms to integrate all of the above that can account for acute changes in activity levels and/or hormonal changes
Resources/references Insulin Pumps and Continuous Glucose Monitoring: A User s guide to Effective Diabetes Management. Francine R. Kaufman, MD with Emily Westfall 2012 Pumping Insulin. John Walsh, PA, CDTC and Ruth Roberts, MA. 5th edition 2013 Think Like a Pancreas. Gary Scheiner, MS, CDE. Revised edition 2011 Tanenberg: The Insulin Pump Book, MiniMed 1995: 21-30 www.medtronicdiabetes.com www.myomnipod.com www.tandemdiabetes.com www.dexcom.com www.bionicpancreas.org www.eversensediabetes.com http://integrateddiabetes.com/2018-new-insulin-pump- comparisons-and-reviews/
Disclosure to Participants Notice of Requirements For Successful Completion Please refer to learning goals and objectives Learners must attend the full activity and complete the evaluation in order to claim continuing education credit/hours Conflict of Interest (COI) and Financial Relationship Disclosures: Mindy Saenz, RDN, LDN, CDE has no COI or financial disclosures. Non-Endorsement of Products: Accredited status does not imply endorsement by AADE, ANCC, ACPE or CDR of any commercial products displayed in conjunction with this educational activity Off-Label Use: Participants will be notified by speakers to any product used for a purpose other than for which it was approved by the Food and Drug Administration.