Understanding Heat Energy Transfer and Conduction in Materials
Heat energy is a form of energy measured in joules that causes particles to move faster, expand, and change states. Learn about the difference between heat and temperature, heating and cooling processes, and the methods of energy transfer such as conduction. Discover how materials conduct heat differently and explore the concepts of conduction, conductors, and their practical applications.
Uploaded on Sep 26, 2024 | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
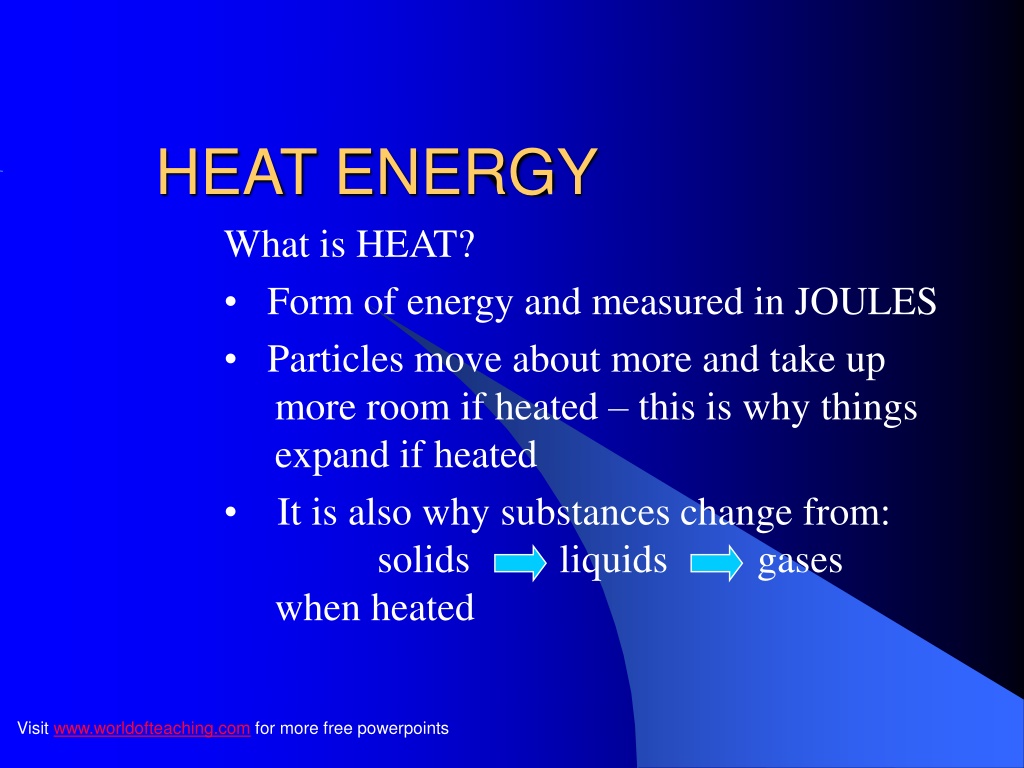
HEAT ENERGY What is HEAT? Form of energy and measured in JOULES Particles move about more and take up more room if heated this is why things expand if heated It is also why substances change from: solids liquids gases when heated Visit www.worldofteaching.com for more free powerpoints
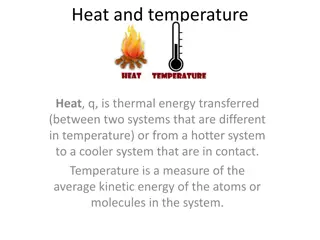
Heat and Temperature The temperature of an object tells us how HOT it is Measured in degrees Celsius - C It is NOT the same as heat energy although the two quantities are related. e.g. a beaker of water at 60 C is hotter than a bath of water at 40 C BUT the bath contains more joules of heat energy
Heating and Cooling If an object has become hotter, it means that it has gained heat energy. If an object cools down, it means it has lost energy
Heating and Cooling cont Heat energy always moves from: HOT object COOLER object e.g.Cup of water at 20 C in a room at 30 C - gains heat energy and heats up its temperature rises Cup of water at 20 C in a room at 10 C loses heat energy and cools down its temperature will fall.
HEAT ENERGY Energy transfer Conduction Convection Radiation
Conduction Heat is transferred through a material by being passed from one particle to the next Particles at the warm end move faster and this then causes the next particles to move faster and so on. In this way heat in an object travels from: the HOT end the cold end
Conduction cont Occurs by the particles hitting each other and so energy is transferred. Can happen in solids, liquids and gases, Happens best in solids-particles very close together Conduction does not occur very quickly in liquids or gases
Conductors Materials that conduct heat quickly are called conductors All metals are good conductors of heat Copper is a very good conductor of heat Pans for cooking are usually made with a copper or aluminium bottom and plastic handles
Insulators/poor conductors Materials that conduct heat slowly or poorly are called insulators Glass, wood, plastic and rubber are poor conductors (good insulators) Nearly all liquids including water are poor conductors (good insulators) Gases, including air are poor conductors,e.g., wool feels warm because it traps a lot of air A fridge has insulation material round it to keep it cold reduces amount of heat conducted to inside from the warmer room
Convection Takes place in material where particles can move around inside the material, i.e. liquid or gas The heat is carried by the particles themselves moving Convection currents Occur because an area with warm particles expands and becomes less dense than the cooler areas nearby. The warm area rises. Cooler particles fall into the space left by the warm particles and convection current is set up
Convection Currents Hot liquids and gases expand and rise while the cooler liquid or gas falls 2. Goes across 3. Then down 1. Hot air rises 4. And across
Convection cont The sun can cause large convection currents - WINDS During daytime the land warms up more than the sea. The warm air rises over the land and cool air falls over the sea. So we feel a sea breeze. Rising convection currents can be uses by glider pilots to keep their planes in the air and by birds to stay aloft.
Radiation Transfer of heat directly form the source to the object by a wave, travelling as rays. Heat radiation is also known as INFRA-RED RADIATION All objects that are hotter than their surroundings give out heat as infra-red radiation Heat transfer by radiation does not need particles to occur and is the only way energy can be transferred across empty space
Emitters Hotter objects emit (give out) heat Different surfaces emit heat at different speeds A dull black surfaces loses energy more quickly it is a good radiator A bright shiny or white surface is a poor radiator Marathon runners need to keep warm at the end of races, covering in shiny blankets reduces radiation and therefore heat loss.
Emitters of heat Bright shiny can Poor radiator Dull black can Good Radiator
Absorbers Cooler objects absorb (take in) heat Substances absorb heat at different speeds Dull, black surfaces absorb heat quickly Bright, shiny surfaces absorb heat slowly In hot countries, people wear bright white clothes and paint their houses white to reduce absorption of energy from the sun. Petrol storage tanks sprayed silver to reflect sun s rays
Absorbers Shiny, bright can Poor absorber Dull black can Good absorber
Key Words Temperature Cold Insulator Radiation Heat Transfer Convection Conduction Conductor Absorber Emitter