Understanding Free Ion Spectroscopic Terms and Their Splittings
Dive into the world of free ion spectroscopic terms and their splitting in different ligand fields. Explore configurations, energy levels, and Orgel diagrams to grasp the complexities of electron-electron interactions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
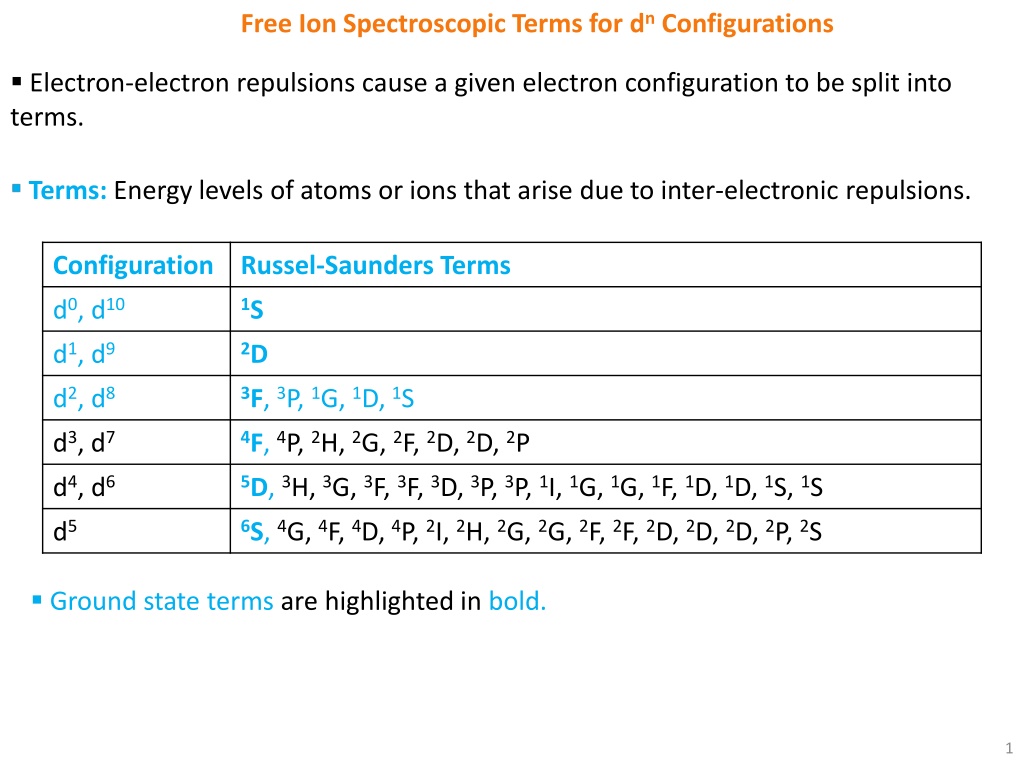
Free Ion Spectroscopic Terms for dn Configurations Electron-electron repulsions cause a given electron configuration to be split into terms. Terms: Energy levels of atoms or ions that arise due to inter-electronic repulsions. Configuration Russel-Saunders Terms d0, d10 1S d1, d9 2D d2, d8 3F, 3P, 1G, 1D, 1S d3, d7 4F, 4P, 2H, 2G, 2F, 2D, 2D, 2P d4, d6 5D,3H, 3G, 3F, 3F, 3D, 3P, 3P, 1I, 1G, 1G, 1F, 1D, 1D, 1S, 1S d5 6S, 4G, 4F, 4D, 4P, 2I, 2H, 2G, 2G, 2F, 2F, 2D, 2D, 2D, 2P, 2S Ground state terms are highlighted in bold. 1
Splitting of Free Ion Terms In Octahedral Ligand Field Term Components Formed by Splitting in Octahedral Ligand Field S A1g T1g Eg+T2g A2g+T1g+T2g A1g+Eg+T1g+T2g Eg+T1g+T2g+T2g A1g+A2g+Eg+T1g+T2g+T2g P D F G H I s (spherically symmetrical) and p orbitals do not split in octahedral ligand field. Thus, S and P terms will be unaffected and they give A1 and T1 terms, respectively. Orbital multiplicity of the free ion terms is retained in the aggregate of component terms. Thus, an F term (L = 3, orbital multiplicity = 7) splits into one singlet (A2) and two triplet (T1 and T2) terms, maintaining a total orbital degeneracy of (1+3+3) or 7. Spin multiplicity of the term and its components are same becausethe spin state of an electron is not disturbed by the symmetry (Oh, Td, etc.) of ligand field. 2
Splitting of D and F Terms in Tetrahedral and Octahedral Symmetries Hole concept suggests an inversion of term splitting between dn and d10-n configurations. For example, a d9metal ion has an electron vacancy or hole in its d level and thus can be regarded as the inverse of a d1 arrangement. An inversion of term splitting also occurs between tetrahedral and octahedral symmetries. Since ligand fields of these two symmetries produce inverse splitting patterns for d orbitals, any free ion term will also split into new terms by tetrahedral and octahedral fields, but the energy ordering will be opposite for two symmetries. 3
Orgel Diagrams Orgel diagrams show the energies of terms as a function of ligand field strength. With the help of Orgel diagrams, the number of spin allowed absorption bands can be predicted in the UV/Visible spectrum of a complex. Orgel Diagram for Free Ion D Ground State From this diagram, we can see why one absorption is observed in the electronic spectra of d1, d4, d6 and d9 octahedral and tetrahedral complexes. 4
Orgel Diagram for Free Ion F Ground State 0 From this diagram, we can see why three absorptions are observed in the electronic spectra of d2, d3, d7 and d8 octahedral and tetrahedral complexes. Labels on Orgel diagrams do not include spin multiplicity designations. Spin multiplicities must be added when discussing specific transitions. 5
Orgel Diagram for Free Ion F Ground State At increased field strengths, the lines describing the T1g(F) and T1g(P) terms curve repel away from one another; there is interaction between terms of the same symmetry and they are not allowed to cross (non-crossing rule). The condition of no interaction is shown by dashed lines. Electronic Spectra of 3d Transition Metal Complexes [M(H2O)6]2+ 6
Electronic Spectra of 3d Transition Metal Complexes [M(H2O)6]2+ 7