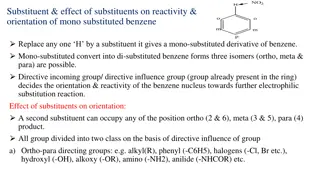
Understanding Displacement Reactions and Reactivity Series
Explore the concept of displacement reactions and reactivity series in chemistry. Learn how to predict if a pair of substances will undergo a displacement reaction and identify the more reactive metal in a pair. Observations and examples involving copper, magnesium, zinc, iron, and calcium are provided.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
* ** *** Describe what you can see happening when a displacement reaction happens Use the reactivity series to predict if a pair of substances will undergo a displacement reaction State which metal is more reactive in a pair of metals * ** *** Describe what you can see happening when a displacement reaction happens State which metal is more reactive in a pair of metals Use the reactivity series to predict if a pair of substances will undergo a displacement reaction
Add solid metal powder or lumps to test tube Liquid reactant in test tube * ** *** Describe what you can see happening when a displacement reaction happens State which metal is more reactive in a pair of metals Use the reactivity series to predict if a pair of substances will undergo a displacement reaction
What did you see happen? Which metal is the most reactive? Chemical 1 Chemical 2 copper chloride magnesium copper magnesium iron copper chloride iron copper copper chloride zinc copper zinc zinc nitrate magnesium zinc magnesium * ** *** Describe what you can see happening when a displacement reaction happens (L3) copper State which metal is more reactive in a pair of metals (L4) zinc nitrate Use the reactivity series to predict if a pair of substances will undergo a displacement reaction (L5) copper zinc magnesium calcium chloride magnesium calcium iron magnesium hydroxide iron magnesium calcium chloride copper calcium copper * ** *** Describe what you can see happening when a displacement reaction happens State which metal is more reactive in a pair of metals Use the reactivity series to predict if a pair of substances will undergo a displacement reaction
copper magnesium zinc iron calcium * ** *** Describe what you can see happening when a displacement reaction happens State which metal is more reactive in a pair of metals Use the reactivity series to predict if a pair of substances will undergo a displacement reaction
Iron vs copper Calcium vs copper Magnesium vs iron Zinc vs copper Calcium vs zinc * ** *** Describe what you can see happening when a displacement reaction happens State which metal is more reactive in a pair of metals Use the reactivity series to predict if a pair of substances will undergo a displacement reaction
Predict if there would be a displacement reaction: 1. Copper sulfate and magnesium 2. Magnesium nitrate and calcium 3. Zinc chloride and copper 4. Copper and calcium chloride 5. Copper sulfate and iron * ** *** Describe what you can see happening when a displacement reaction happens State which metal is more reactive in a pair of metals Use the reactivity series to predict if a pair of substances will undergo a displacement reaction