Understanding Allylic Strain in Organic Chemistry
Allylic strain refers to the unfavorable nonbonding repulsion caused by certain substituents in the allylic position of a molecule. This strain can impact reaction outcomes and diastereoselectivity, as seen in examples like Diels-Alder and hydroboration oxidation reactions. Proper understanding and management of allylic strain are crucial in physical organic chemistry studies.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Allylic Strain Organic Pedagogical Electronic Network Created by Sophia Robinson Physical Organic Chemistry I CHEM 7240 (Sigman), 2015
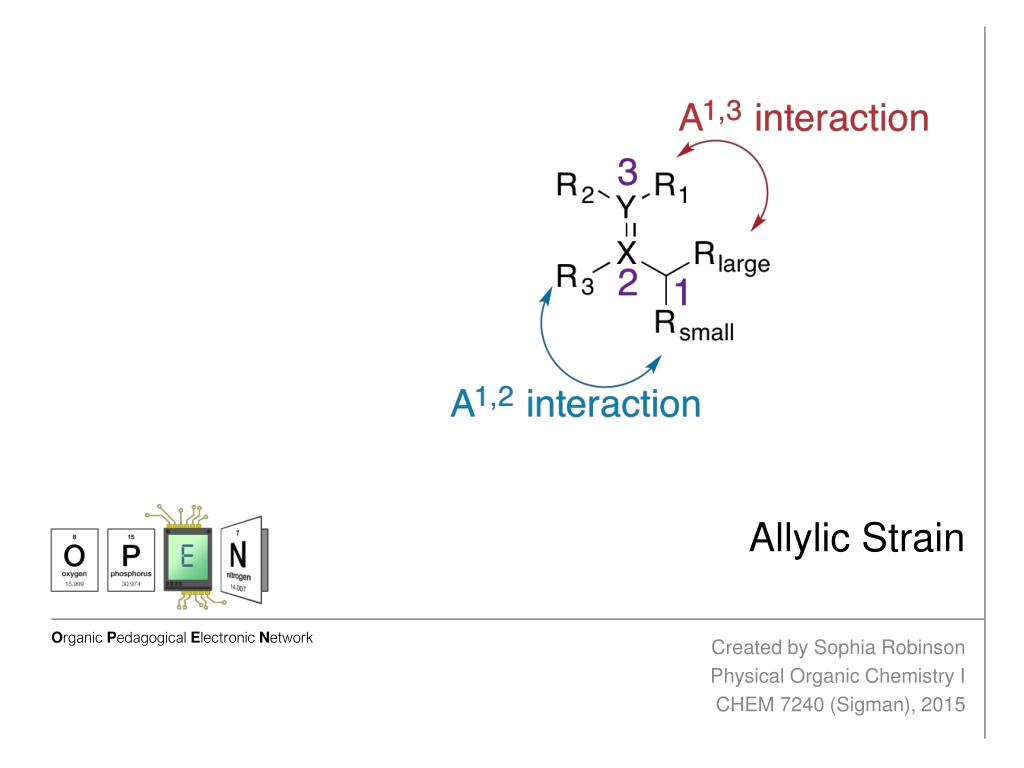
Allylic Strain The allylic position is the atom bound to a double bonded atom. The substituents on the allylic carbon and the doubly bonded atoms can result in allylic strain. Allylic strain arises from eclipsed conformations when Z allylic substituents and Z substituents at the 2- or 3-positions are large enough to create an unfavorable nonbonding repulsion. Strain between the allylic and 2-position substituents is called A1,2- strain (2.7 kcal/mol). Strain between the allylic and 3-position substituents is called A1,3-strain (3.9 kcal/mol). A1,2-strain and A1,3-strain affect the diastereoselectivity of reactions. http://isites.harvard.edu/fs/docs/icb.topic93502.files/Lectures_and_Handouts/05-Conformational_Anal-2.pdf Hoffman, R.W. Chem Rev. 1989, 89, 1841
Allylic Strain Consider the general structure where X and Y are C, N, or O: The allylic center and its substituents often lead to a significant steric bias toward reactions occurring at the double bond. http://isites.harvard.edu/fs/docs/icb.topic93502.files/Lectures_and_Handouts/05-Conformational_Anal-2.pdf Hoffman, R.W. Chem Rev. 1989, 89, 1841
Allylic Strain Consider rotation about the bond of the two substituted allylic systems shown: These values are from ab intio calculations performed by Houk. A and B are both minima in conformational energy while C is not an energy minimum. When the C(3) substituent is a methyl rather than hydrogen, conformational equilibrium strongly favors D. With the methyl present, E is destabilized by allylic 1,3- strain and is an energy maximum. F is an energy minimum but is far less energetically favorable than D. Hoffman, R.W. Chem Rev. 1989, 89, 1841
Examples of Reactions influenced by Allylic Strain Diels-Alder Two transition states for the Diels-Alder reaction are shown in which opposite faces of the diene are shielded: The diastereoselectivity of a Diels-Alder reaction is increased by presence of an additional substituent on carbon 2 as the substituent destabilizes transition state B through A1,3-strain. Hoffman, R.W. Chem Rev. 1989, 89, 1841
Examples of Reactions influenced by Allylic Strain Hydroboration Oxidation The alkene aligns itself as shown such that the smallest of the substituents is staggered with the alkene double bond to prevent A1,3-strain. The borane then approaches from the side of the medium-sized methyl substituent rather than the larger R1 substituent: Houk, K.N.; Rondan, N.G.; Wu, Y.D.; Metz, JT; Paddon-Row, M.N.; Tetrahedron, 1984, 40, 2257
Problems Hoffman, R.W. Chem Rev. 1989, 89, 1841 Fleming, I and coworkers, Chem. Commun.1985, 318;
Solutions 1.) R = Me, A is major diastereomer (87 A : 13 B) R = Et, A is major diastereomer (80 A : 20 B) R = CHMe2, B is major diastereomer (40 A : 60 B) 2.) B. B is the most stable, C is the least stable 3.) B (> 95% ds) (A ds = 50 %) 4.) B. B is major diastereomer. If SiMe3 substituent is not present, no diastereoselectivity is observed 5.) C (ds = 90%)
Contributed by: Sophia Robinson, (Undergraduate) Physical Organic Chemistry I CHEM 7240 (Sigman), University of Utah, 2015 This work is licensed under a Creative Commons Attribution- ShareAlike 4.0 International License.