Understanding Active Transport of Molecules: Driven by ATP Hydrolysis
Active transport, fueled by ATP hydrolysis, facilitates the movement of molecules against their concentration gradients, essential for processes like ion pumping across membranes. The Na+-K+ pump, a prime example, utilizes ATP to transport Na+ and K+ ions across cellular membranes, maintaining important gradients critical for nerve signaling, osmotic balance, and cell volume regulation.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
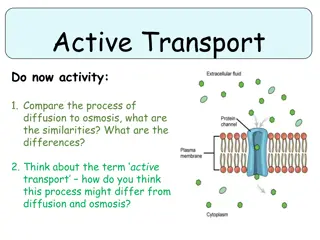
Active Transport Driven by ATP Hydrolysis The net flow of molecules by facilitated diffusion, through either carrier proteins or channel proteins, is always energetically downhill in the direction determined by electrochemical gradients across the membrane. In many cases the cell must transport molecules against their concentration gradients. In active transport, energy provided by another coupled reaction (such as the hydrolysis of ATP) is used to drive the uphill transport of molecules in the energetically unfavorable direction.
The ion pumps responsible for maintaining gradients of ions across the plasma membrane provide important examples of active transport driven directly by ATP hydrolysis. The concentration of Na+is approximately ten times higher outside than inside of cells, whereas the concentration of K+is higher inside than out. These ion gradients are maintained by the Na+-K+pump (also called the Na+-K+ ATPase), which uses energy derived from ATP hydrolysis to transport Na+and K+ against their electrochemical gradients. This process is a result of ATP-driven conformational changes in the pump.
First, Na+ions bind to high-affinity sites inside the cell. This binding stimulates the hydrolysis of ATP and phosphorylation of the pump, inducing a conformational change that exposes the Na+-binding sites to the outside of the cell and reduces their affinity for Na+. Consequently, the bound Na+is released into the extracellular fluids. At the same time, high-affinity K+-binding sites are exposed on the cell surface.
The binding of extracellular K+to these sites then stimulates hydrolysis of the phosphate group bound to the pump, which induces a second conformational change, exposing the K+-binding sites to the cytosol and lowering their binding affinity so that K+is released inside the cell. The pump has three binding sites for Na+and two for K+, so each cycle transports three Na+and two K+across the plasma membrane at the expense of one molecule of ATP.
One critical role of the Na+and K+gradients established by the pump is the propagation of electric signals in nerve and muscle. The Na+gradient established by the pump is also utilized to drive the active transport of a variety of other molecules. Another important role of the Na+-K+pump in most animal cells is to maintain osmotic balance and cell volume. The cytoplasm contains a high concentration of organic molecules, including macromolecules, amino acids, sugars, and nucleotides.
In the absence of a counterbalance, this would drive the inward flow of water by osmosis, which if unchecked would result in swelling and eventual bursting of the cell. The required counterbalance is provided by the ion gradients established by the Na+-K+ pump. In particular, the pump establishes a higher concentration of Na+outside than inside the cell. The flow of K+through open channels further establishes an electric potential across the plasma membrane. This membrane potential in turn drives Cl-out of the cell, so the concentration of Cl-(like that of Na+) is about ten times higher in extracellular fluids than in the cytoplasm. These differences in ion concentrations balance the high concentrations of organic molecules inside cells, equalizing the osmotic pressure and preventing the net influx of water.
Ion gradients across the plasma membrane of a typical mammalian cell The concentrations of Na+and Cl-are higher outside than inside the cell, whereas the concentration of K+is higher inside than out. The low concentrations of Na+and Cl-balance the high intracellular concentration of organic compounds, equalizing the osmotic pressure and preventing the net influx of water.
The active transport of Ca2+across the plasma membrane is driven by a Ca2+ pump that is structurally related to the Na+-K+pump and is similarly powered by ATP hydrolysis. The Ca2+pump transports Ca2+out of the cell, so intracellular Ca2+ concentrations are extremely low: approximately 0.1 M, in comparison to extracellular concentrations of about 1 mM. This low intracellular concentration of Ca2+makes the cell sensitive to small increases in intracellular Ca2+levels. Such transient increases in intracellular Ca2+play important roles in cell signaling.
Similar ion pumps in the plasma membranes of bacteria, yeasts, and plant cells are responsible for the active transport of H+out of the cell. In addition, H+is actively pumped out of cells lining the stomach, resulting in the acidity of gastric fluids. Structurally distinct pumps are responsible for the active transport of H+ into lysosomes and endosomes.
The largest family of membrane transporters consists of the ABC transporters, so called because they are characterized by highly conserved ATP-binding domains or ATP-binding cassettes. More than a hundred family members have been identified in both prokaryotic and eukaryotic cells. In bacteria, ABC transporters utilize energy derived from ATP hydrolysis to transport a wide range of molecules, including ions, sugars, and amino acids. In eukaryotic cells, the first ABC transporter was discovered as the product of a gene (called the multidrug resistance, or mdr, gene) that makes cancer cells resistant to a variety of drugs used in chemotherapy.
Two MDR transporters have now been identified. They are normally expressed in a variety of cells, where they function to remove potentially toxic foreign compounds. For example, expression of an MDR transporter in capillary endothelial cells of the brain appears to play an important role in protecting the brain from toxic chemicals. Unfortunately, the MDR transporters are frequently expressed at high levels in cancer cells where they recognize a variety of drugs and pump them out of cells, thereby making the cancer cells resistant to a broad spectrum of chemotherapeutic agents and posing a major obstacle to successful cancer treatment.
Structure of an ABC transporter The basic structural unit of ABC transporters consists of six transmembrane domains followed by an ATP-binding cassette. In plasma membrane transporters (such as the MDR transporter), two of these units are fused so that the transporter contains 12 transmembrane domains and two ATP-binding sites.
Another medically important member of the ABC transporter family is the gene responsible for cystic fibrosis. Although it is a member of the ABC family, the product of this gene (called the cystic fibrosis transmembrane conductance regulator, or CFTR) functions as a Cl-channel in epithelial cells, and defective Cl-transport is characteristic of the disease. The CFTR Cl-channel is also unusual in that it appears to require both ATP hydrolysis and cAMP-dependent phosphorylation in order to open. The structural basis for the function of CFTR as a regulated ion channel remains to be elucidated by future research.
Active Transport Driven by Ion Gradients The ion pumps and ABC transporters utilize energy derived directly from ATP hydrolysis to transport molecules against their electrochemical gradients. Other molecules are transported against their concentration gradients using energy derived not from ATP hydrolysis but from the coupled transport of a second molecule in the energetically favorable direction. The Na+gradient established by the Na+-K+pump provides a source of energy that is frequently used to power the active transport of sugars, amino acids, and ions in mammalian cells. The H+gradients established by the H+pumps of bacteria, yeast, and plant cells play similar roles.
The epithelial cells lining the intestine provide a good example of active transport driven by the Na+gradient. These cells use active-transport systems in the apical domains of their plasma membranes to take up dietary sugars and amino acids from the lumen of the intestine. The uptake of glucose, for example, is carried out by a transporter that coordinately transports two Na+and one glucose into the cell.
The flow of Na+down its electrochemical gradient provides the energy required to take up dietary glucose and to accumulate high intracellular glucose concentrations. Glucose is then released into the underlying connective tissue (which contains blood capillaries) at the basolateral surface of the intestinal epithelium, where it is transported down its concentration gradient by facilitated diffusion. The uptake of glucose from the intestinal lumen and its release into the circulation thus provides a good example of the polarized function of epithelial cells, which results from the specific localization of active transport and facilitated diffusion carriers to the apical and basolateral domains of the plasma membrane, respectively.
Active transport of glucose Active transport driven by the Na+gradient is responsible for the uptake of glucose from the intestinal lumen. The transporter coordinately binds and transports one glucose and two Na+into the cell. The transport of Na+in the energetically favorable direction drives the uptake of glucose against its concentration gradient.
Glucose transport by intestinal epithelial cells A transporter in the apical domain of the plasma membrane is responsible for the active uptake of glucose (by cotransport with Na+) from the intestinal lumen. As a result, dietary glucose is absorbed and concentrated within intestinal epithelial cells. Glucose is then transferred from these cells to the underlying connective tissue and blood supply by facilitated diffusion, mediated by a transporter in the basolateral domain of the plasma membrane. The uptake of glucose from the digestive tract and its transfer to the circulation is dependent on the restricted localization of glucose transporters mediating active transport and facilitated diffusion to the apical and basolateral domains of the plasma membrane, respectively.
The coordinate uptake of glucose and Na+is an example of symport, the transport of two molecules in the same direction. In contrast, the facilitated diffusion of glucose is an example of uniport, the transport of only a single molecule. Active transport can also take place by antiport, in which two molecules are transported in opposite directions. For example, Ca2+is exported from cells not only by the Ca2+pump but also by an Na+-Ca2+antiporter that transports Na+into the cell and Ca2+out.
Examples of antiport Ca2+and H+are exported from cells by antiporters, which couple their export to the energetically favorable import of Na+.