Understanding Absorption Processes and McCabe-Thiele Analysis
Desirable conditions in designing an absorption process include low pressure and high temperature for the gas in and liquid out. Learn about appropriate plots, points on the operating line, absorption factors, minimum absorbent usage, assumptions in McCabe-Thiele analysis, and differences between counter-current and cross-flow absorption processes.
- Absorption processes
- McCabe-Thiele analysis
- Gas-liquid absorption
- Equilibrium modeling
- Process engineering
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Which is desirable in designing an absorption process? gas out liquid in A. high pressure, high temperature B. high pressure, low temperature C. low pressure, high temperature gas in D. low pressure, low temperature liquid out
Which of the following is an appropriate plot for a physical absorption process for a dilute species? The black line is the operating line and the blue line is the equilibrium line. Vapor mol fraction 0 Vapor mol fraction B A 0 Liquid mol fraction Liquid mol fraction Vapor mol fraction C D Vapor mol fraction 0 0 Liquid mol fraction Liquid mol fraction
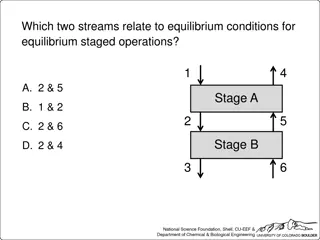
For this physical absorption process, which point(s) lie(s) on the operating line for the McCabe-Thiele graphical solution? (Note: x and y represent liquid and gas mole fractions, respectively) G, y1 S, x0 A. (xN, y1) B. (x0, y1) C. (x0, yN+1) D. (xN, yN+1) E. B and D S, xN G, yN+1
A dilute species is being absorbed. Equilibrium is modeled using Henry s law. Which has the greatest absorption factor (A = L/KV)? operating line = black; equilibrium line = blue Vapor mol fraction 0 Vapor mol fraction A B 0 Liquid mol fraction Liquid mol fraction Vapor mol fraction Vapor mol fraction D C 0 0 Liquid mol fraction Liquid mol fraction
A process calls for 98% recovery of solute B from a vapor stream using absorption. The current design (100 L) can accomplish this, but an engineer wants to use less absorbent. What is the minimum amount of liquid absorbent (L) that can be used assuming the number of stages are not limiting? Vapor mol fraction A. 50 B. 33 C. 25 D. 10 E. < 10 Liquid mol fraction
Several assumptions are made during McCabe-Thiele analysis of an absorber including constant molar overflow and an isothermal system. What additional assumption is made for an analytical solution for dilute systems? A. Murphree efficiencies for every stage are 1. B. The solvent stream is fed as a pure liquid/vapor. C. The equilibrium line is linear. D. No additional assumptions are made.
For a fixed purity/yield, counter-current absorption processes require __________ solvent than cross-flow processes. A. less B. more C. dependent on the scenario
For a fixed solvent quantity, counter-current absorption processes can achieve ________ purity and yield than cross-flow processes. A. higher B. lower C. dependent on the scenario
For absorption and stripping processes, appropriate solutions can always be obtained by treating the system as concentrated as opposed to dilute (< 1 wt%). A. true B. false
What is heat exchanger B doing? treated gas B gas to be treated stripping gas A spent solvent A. heating B. cooling C. nothing (not needed) D. depends
What is heat exchanger A doing? treated gas B gas to be treated stripping gas A spent solvent A. heating B. cooling C. nothing (not needed) D. depends
The absorption factor, AN, for a given stage and component for this column is shown below: ?? ??= liquid L1 vapor V1 ???? where K is the thermodynamic K-value and N represents the stage #. What is the relationship between A and the stripping factor, S? 1 2 3 N-2 N-1 N A. AN = SN B. AN = 1/SN C. AN = S1 D. AN = 1/S1 vapor VN+1 liquid LN
The recovery factor, A, for an absorption column is shown below: ?1= ??+1?? liquid L1 vapor V1 where is the molar flow rate of the absorbed species. A perfect separation means A is _____. 1 2 3 N-2 N-1 N A. 0 B. 0.5 vapor VN+1 liquid LN C. 1 D. Infinity
For this absorption column, which of the following must be true? Y1, V X0, L A. VLE reached at each stage 1 B. only one solute absorbed C. absorbent not vaporized n D. absorbate prefers liquid phase N E. all of the above YN+1, V XN, L