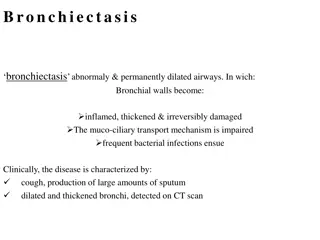
The CLEAR Trial: Investigating Hypertonic Saline and Carbocisteine for Bronchiectasis Treatment
The CLEAR Trial is a 2x2 factorial randomized trial assessing the clinical and cost-effectiveness of hypertonic saline and carbocisteine for airway clearance in bronchiectasis patients over 52 weeks. Led by Professor Stuart Elborn and Professor Judy Bradley, the trial involves randomization, sealed envelope processes, and patient information management through an online system.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
The CLEAR The CLEAR Trial A 2x2 factorial randomised open label trial to determine the clinical and cost- effectiveness of hypertonic saline (HTS 6%) and carbocisteine for airway clearance versus usual care over 52 weeks in bronchiectasis (BE) Trial Chief Investigator: Professor Stuart Elborn, Queen s University Belfast Lead Applicant Physiotherapist: Professor Judy Bradley, Queen s University Belfast CLEAR_Site Initiation Training PART 6_Final v12.0 03/08/2021
Site Initiation Training Part 6: Site Initiation Training Part 6: Randomisation Process Randomisation Process - - CTU CTU
Randomisation Randomisation Randomisation takes place at the baseline visit after all the baseline assessments, questionnaires and spirometry have been completed and confirmation of eligibility has been documented.
Sealed Envelopes Randomisation Sealed Envelopes Randomisation After staff log in with their password and email address you will see this screen. To enter the Access with role: Investigator button
Sealed Envelopes Randomisation Sealed Envelopes Randomisation This is the screen that you will see when you have accessed the system From here you can select: Randomise to randomise a patient to an intervention Randomisations view a list of all randomised patients at your site & create a query about any patient randomised at your site Queries view all open and closed queries raised at your site
Sealed Envelopes Randomisation Sealed Envelopes Randomisation This is the Randomise tab Here you will enter the patients information After you have entered in all the information you will be asked to enter your e-signature (password) to sign off the investigators declaration If the patient is eligible an email will be sent to all site email accounts receiving notifications Please note: If a patient takes seasonal Macrolides, but is currently in their seasonal break, please select Yes to Currently taking Macrolides
Sealed Envelopes Randomisation Sealed Envelopes Randomisation If you click edit query you will see the information validated by the administrator If necessary the investigator can take further action.
Sealed Envelopes Randomisation Sealed Envelopes Randomisation View in the Randomisation tab By selecting on a Subject ID you can raise a query for an administrator in the NICTU to action