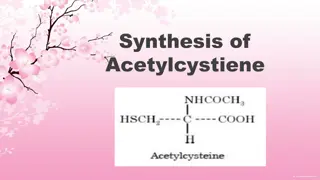
Synthesis and Purification of Acetaminophen (Paracetamol) for Analgesic Properties
Acetaminophen, a widely used analgesic and anti-pyretic agent, is synthesized and purified in this experiment. The process involves suspending P-aminophenol in water, reacting with acetic anhydride, filtering, crystallizing, and collecting the product. The compound's mechanism of action, chemical properties, and purification techniques such as crystallization, extraction, chromatography, and filtration are discussed. Calculations for yield and purity evaluation are also included.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Experiment Five Synthesis and purification of the analgesic agent, Acetaminophen (Paracetamol)
Introduction: Acetaminophen is a non-steroidal anti- inflammatory/analgesic agent, it has also anti-pyretic properties. It is widely used in arthritic and rheumatoid conditions involving musculoskeletal pain and other disorders such as headache. 200px-Paracetamol-from-xtal-3D-balls 200px-Paracetamol-skeletal
Mechanism of action Now, recent research (2) has shown the presence of a new, previously unknown cyclooxygenase enzyme COX-3, found in the brain and spinal cord, which is selectively inhibited by paracetamol, and is distinct from the two already known cyclooxygenase enzymes COX-1 and COX-2. It is now believed that this selective inhibition of the enzyme COX-3 in the brain and spinal cord explains the effectiveness of paracetamol in relieving pain and reducing fever without having unwanted gastrointestinal side effects.
Chemical properties: White crystalline powder. Molecular weight = 151.17 g/mole. Melting point = 170 C. Insoluble in water, very soluble in ethanol.
Synthetic procedure: Suspend 2.2 g of P-aminophenol in 6 ml distilled water. Warm the mixture in a water bath until all solid dissolved. Add 2.5 ml acetic anhydride to the hot solution, and then heat the reaction in water bath for 10 minutes. Filter while the solution is hot using Buchner filtration unit. Leave to cool down for 10 minutes. Filter the solid precipitate, and then wash it with cold water. Re-dissolve in about 20 ml hot distilled water .filter while hot cool in ice bath to form crystals. Collect the crystals by Buchner filtration, and then dry it in the oven.
Calculations Weigh and then calculate the % yield of the reaction. p-aminophenol,Mwt=109.13 g/mole Acetic acid anhydrous, d=1.082 g/ml, Mwt=102.09 g/mole Molecular weight of paracetamol= 151.17 g/mole.
Purity Definition is the absence of impurityor contaminants in a substance
Purification technique: Crystalization Extraction Chromatography Filtration distillation
Technique to measure purity mp HPLC TLC NMR Mass spectroscopy
Identification test Test 1: Dissolve 0.1 g paracetamol in 10 ml water Add 0.05 ml ferric chloride solution. Violet blue color is produced Test 2: Boil 0.1 g of paracetamol with 1 ml HCl for 3 min. Add 10 ml water & cool, no ppt produced. Add 0.05 ml 0.1N potassium dichromate. A violet colour slowly develops, which don t become red .
Assay is done usually by using: Spectrophotometric Method. HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC).
What is the class of Paracetamol ? What is the difference between Panadol, Panadol extra, Panadol night , Panadol cold & flu ,Panadol advance & Panadol joint? How to prepare acetaminophen from benzene? Why it is better to start with p-aminophenol to synthesis acetaminophen? Why acetic anhydride must not be in excess? Why the percent yield could be very low?