Reactor Design Reviews for Nonisothermal Systems
Explore nonisothermal reactor design concepts, including total energy balance equations, conversion calculations, application to CSTRs and PFRs, and solving for various parameters using theoretical and numerical methods.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
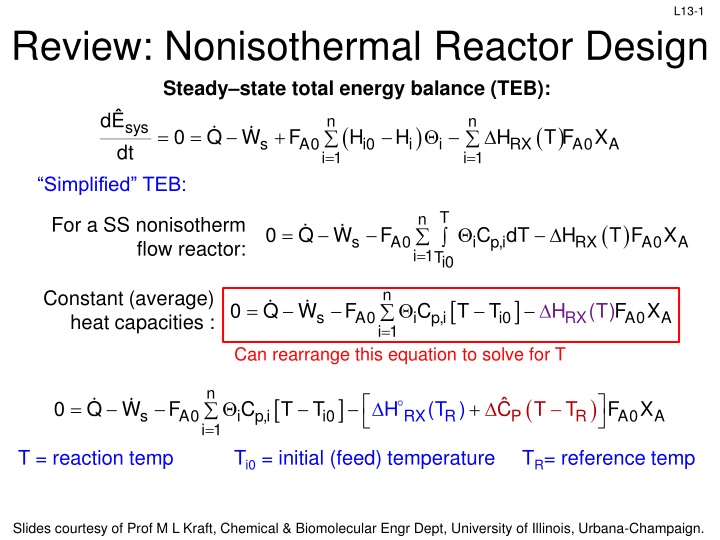
L13-1 Review: Nonisothermal Reactor Design Steady state total energy balance (TEB): dE 0 Q W F H dt Simplified TEB: n n sys ( ) ( ) T F = = + i H H X s A0 i0 i RX A0 A = i 1 = i 1 T n For a SS nonisotherm flow reactor: ( ) T F = 0 Q W F i p,i C dT H X s A0 RX A0 A = i 1Ti0 n Constant (average) heat capacities : = 0 Q W F i p,i C T T H (T)F X s A0 i0 RX A0 A = i 1 Can rearrange this equation to solve for T n ( ) = + 0 Q W F i p,i C T T H (T ) C T T F X s A0 i 0 RX R P R A0 A = i 1 T = reaction temp Ti0 = initial (feed) temperature TR= reference temp Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-2 Review: Solve TEB for Conversion n s A0 i p,i i0 i 1 = ( ) = + 0 Q W F C T T H (T ) C T T F X RX R P R A0 A n Solve for XA: i p,i C + F T T W Q A0 i0 s i 1 = = X A ( ) + H (T ) C T T F RX R P R A0 Plug in Q for the specific type of reactor For an adiabatic reaction (Q=0) and shaft work can be neglected ( S=0) n i p,i C T T i0 i 1 = = X A ( ) + H (T ) C T T RX R P R T = reaction temp Ti0 = initial (feed) temperature TR= reference temp Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-3 Review: Application to CSTR Case 1: Given FA0, CA0, A, E, Cpi, H I, and XA, calculate T & V a) b) c) Solve TEB for T at the exit (Texit = Tinsidereactor) Calculate k = Ae-E/RT where T was calculated in step a Plug the k calculated in step b into the design equation to calculate VCSTR Case 2: Given FA0, CA0, A, E, Cpi, H I, and V, calculate T & XA a) b) Solve TEB for T as a function of XA (make a table of T vs XA using EB) Solve CSTR design equation for XA as a function of T (plug in k = Ae-E/RT ) (use design eq to make a table of XA vs T) Plot XA,EB vs T & XA,MB vs T on the same graph. The intersection of these 2 lines is the conditions (T and XA) that satisfies the energy & mass balance XA,EB = conversion determined from the TEB equation XA,MB = conversion determined using the design equation c) XA,exit Intersection is T and XA that satisfies both equations XA,MB XA XA,EB T Texit Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-4 Review: Application to a SS PFR FA0 FA XA T distance Negligible shaft work ( S=0) and adiabatic (Q=0) a) b) c) d) Use TEB to construct a table of T as a function of XA Use k = Ae-E/RT to obtain k as a function of XA Use stoichiometry to obtain rA as a function of XA Calculate: XA A0 XA0 May use numerical methods dX A = V F ( ) r X ,T A A Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-5 L13: Equilibrium Conversion in Nonisothermal Reactor Design The highest conversion that can be achieved in reversible reactions is the equilibrium conversion For reversible reactions, the equilibrium conversion is usually calculated first The equilibrium conversion increases with increasing temperature for endothermic reactions The equilibrium conversion decreases with increasing temperature for exothermic reactions Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-6 Review of Equilibrium Kinetics kA kA + + aA b B c C d D Gas-phase reaction: c d products C C raised to stoichiometric coefficients raised to stoichiometric coefficients C C C C KC: equilibrium constant (capital K): C D = K C a b reactants A B ( ) H T If KC is given at a single temperature T2, & CP can be neglected then: 1 1 T ( ) T ( ) RX R R = K K T exp C C 2 T 2 c d P P P KP: equilibrium constant in terms of partial pressures Pi: C D = = K P CRT i i P a b P A B = + d b n c a For ideal gases, KP = KC(RT) n where ( ) 2 ( ) ( ) + H T H T C T T dlnK dT Temp dependence of KP is given by van tHoff s equation: Rx RT Rx R P R P = = 2 RT ( ) H T 1 1 T If CP can be neglected then: ( ) T ( ) RX R R = K K T exp P P 2 T 2 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-7 Equilibrium Conversion XAe 1 endothermic heat + A B XA,e exothermic + A B heat 0 T Example) A B CA0=1 CB0=0 ( ) ( ) A0 Ae C 1 X + C 0 X C C X Rearrange to solve in terms of XAe A0 Ae Be Ae = = K = K C C 1 X Ae Ae ( ) ( ) = 1 K + = + 1 X = K X K X K X K X C Ae C C Ae C Ae C Ae Ae K + This equation enables us to express Xae as a function of T C = X Ae ( ) 1 K C Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-8 XAe and Temperature C K 1 K + ( ) H T 1 1 T ( ) T ( ) RX R R = X = K K T exp Ae ( ) C C 2 T C 2 ( ) H T 1 1 T ( ) RX R R K T exp C 2 T 2 Divide numerator & denominator by KC Substitute for KC: = X Ae ( ) H T 1 1 T ( ) RX R R 1 K + T exp C 2 T 2 1 X = e 1 X e = X Ae ( ) ) Changed sign H T 1 1 T 1 RX R R + exp 1 ( ) K T T C 2 2 ( H T 1 T 1 RX R R Exot hermic: H 0, when T exp and X R X Ae T 2 ( ) H T 1 T 1 RX R R Endo thermic: H 0, when T exp and X R X Ae T 2 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-9 XAe and Temperature X H 1 exp K T 1 = Ae ( ) T 1 T 1 RX R R + 1 ( ) T C 2 2 ( ) H T 1 T 1 ( ) RX R R Exothermic & C =0: H T 0 , when T e xp & X P RX R Ae T 2 + A B heat Makes sense from Le Chatelier s principle Exothermic rxn produces heat increasing temp adds heat (product) & pushes rxn to left (lower conversion) ( ) H T 1 T 1 ( ) RX R R Endothermic & C 0: H T 0 , when T exp & X p R X R Ae T 2 + A heat B Makes sense from Le Chatelier s principle Heat is a reactant in an endothermic rxn increasing temp adds reactant (heat) & pushes rxn to right (higher conversion) Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-10 Adiabatic Equilibrium T Example For the elementary solid-catalyzed liquid-phase reaction A C K B B = r k C A A C 1. 2. Make a plot of equilibrium conversion as a function of temperature. Determine the adiabatic equilibrium temperature and conversion when pure A is fed to the reactor at a temperature of 298 K. = H (298K) 40000 cal/mol A = H (298K) C 60000 cal/mol 50 cal/mol K B = PA = C 50 cal/mol K PB = K 100,000 at 298K e Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-11 = 60000 cal/mol = H (298K) C = 40000 cal/mol H (298K) 50 cal/mol K C A B A B = = 50 cal/mol K K 100000 at 298K P P e A B equilibrium CB Ke C Be K = C = r k C Rate law: Ae A A -rA = 0 e C X ( ) ( ) e K + T A0 1 X e = K (T) e = e ( ) X C e A0 e 1 K T ( ) d lnK dT H e = The Van t Hoff equation: 2 R T ( ) T ( ) T T -C o RX = + H H T C dT R X R p = R = C C 0 p p p B A o o B o = = H H -H -20000 o H 1 T 1 T RX A R X = K K (T )exp - e e 1 R T =298K 1 1 Xe only depends on thermodynamics! Nothing to do with the energy balance! Xe = f (T) Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-12 A B Reaction 50 cal/mol K, & the heat of reaction = 20,000 cal/mol. The energy balance is: n s A i pi i A i = 1 is carried out adiabatically with an inlet temp of 298 K, CPA = Q W F C ( T T )F X H ( T ) C( T T ) Rearrang e + = 0 RX R p R 0 0 0 0 0 n + F i p,i C T T W Q A0 i0 s ( ) T T TR = + C dT H H (T ) = i 1 = X RX RX R P A ( ) + H (T ) C T T F RX R P R A 0 n ( ) C T T n ( ) i pi C T T P 0 i pi C 1 C = 0 A = X p EB i 1 = A ( ) T = X i 1 = H EB RX ( 20000 ( ) T H RX ) 50 T 298 From thermodynamics = X X EB K + C = X Ae ( ) 1 K From energy balance C ( ) C T T P 0 A = X EB ( ) T H RX How to increase the conversion? T Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-13 Does increasing the entering temperature increase XA? p,A A0 C T T H (T ) C K 1 = X Ae ( ) = X H T 1 1 T 1 A,EB RX R R + exp 1 ( ) RX R T T 2 2 XA XA,e at Ti0,1 XA,e at Ti0,2 T (K) Higher T moves XA,EB curve to the right XA,e (Tadiabatic) decreases for an exothermic reaction Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-14 Optimum Feed Temperature For a reversible and exothermic reaction, the feed temperature should be optimized to maximize the conversion High T0: reaction reaches equilibrium fast, but XA is low From thermodynamics XEB XA 0.75 T0 = 500 0.33 T0 = 600 T0 = 350 0.15 600 350 500 W T Low T0 would give high XA,e but the specific reaction rate k is so small that most of the reactant passes through the reactor without reacting (never reach XA,e) There is an optimum inlet temperature! Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
How does one increase XA for adiabatic operation of an exothermic reaction? with interstage cooling! L13-15 XA,EB4 XEB XA,EB3 Each reactor operates adiabatically final conversion XA,EB2 XA,EB1 cooling process T Cooling, C1 C2 C3 T0 Reactor 1 Reactor 2 Reactor 3 Reactor 4 Endothermic reactions are similar, but with heating instead of cooling Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-16 Endothermic Reactions The equilibrium conversion increases with increasing temperature, so use interstage heating to increase the conversion XEB final conversion Red lines are from the energy balance, slant backwards because H RX >0 for endothermic reaction heating process T Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-17 Suppose pure A enters a reactor at 298K . What is the maximum XA achievable in an adiabatic reactor? Assume S=0, and CP = 0, CP.A=60 J/molK, H RX(TR)= 20,000 J/mol, KC=10 exp[2405T-7.2] Solve TEB for XA: 0 Q = 0 n ( ) 0 i p,i C + W F T T H (T ) C T T F X s A0 i0 RX R P R A0 A i 1 = n ( ) i p,i C = + F T T H (T ) C T T F X A0 i0 RX R P R A0 A i 1 = = 1 and A is only sp ecies , solve for X : A A T C T T C T T p,A (T ) A0 = p,A A0 X = = C 0 so X A ( ) P A + H C T H (T ) RX R P R RX R Plot XEB vs T and XA,e vs T to compute the maximum XA graphically 1 = X Ae ( ) H T 1 1 T 1 RX R R + exp 1 ( ) K T T C 2 2 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-18 Suppose pure A enters a reactor at 298K . What is the maximum XA achievable in an adiabatic reactor? Assume S=0, and CP = 0, CP.A=60 J/molK, H RX(TR)= 20,000 J/mol, KC=10 exp[2405T-7.2] p,A A0 A,EB RX R H (T ) C K 1 = X C T T Ae ( ) = X H T 1 1 T 1 RX R R + exp 1 ( ) T T 2 2 T XA,EB 0.294 0.144 0 -0.081 -0.156 -0.231 -0.306 -0.381 -0.456 -0.606 -0.756 -0.906 T XAe 200 250 298 325 350 375 400 425 450 500 550 600 200 250 298 325 350 375 400 425 450 500 550 600 0.000149 0.001634 0.007612 0.014746 0.024722 0.038481 0.056318 0.078262 0.10407 0.165203 0.234315 0.305584 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-19 Suppose pure A enters a reactor at 298K . What is the maximum XA achievable in an adiabatic reactor? Assume S=0, and CP = 0, CP.A=60 J/molK, H RX(TR)= 20,000 J/mol (endothermic), KC=10 exp[2504T-7.2] p,A A0 A,EB RX R H (T ) C K 1 = X C T T Ae ( ) = X H T 1 1 T 1 RX R R + exp 1 ( ) T T 2 2 0.4 0.2 0 XAe 200 300 400 500 600 -0.2 XA -0.4 Nearly 0 conversion, not good! -0.6 -0.8 Energy balance conversion Equilibrium conversion Tadiabatic -1 T (K) Tadiabatic: Outlet T if reactor had an infinite volume XA,e at Tadiabatic is max achievable XA in adiabatic reactor Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-20 Does increasing the inlet temperature to 600K improve the conversion of this reaction? S=0, and CP = 0, CP.A=60 J/molK, H RX(TR)= 20,000 J/mol (endothermic), & KC=10 exp[2504T-7.2] p,A A0 A,EB RX R H (T ) C K 1 = X C T T Ae ( ) = X H T 1 1 T 1 RX R R + exp 1 ( ) T T 2 2 0.4 0.2 XAe 0 200 300 400 500 600 -0.2 Nearly 0 conversion XA Tadiabatic -0.4 -0.6 Energy balance conversion Equilibrium conversion -0.8 -1 T (K) Tadiabatic: Outlet T if reactor had an infinite volume XA,e at Tadiabatic is max achievable XA in adiabatic reactor Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.
L13-21 Does increasing the inlet temperature to 600K improve the conversion of this reaction? S=0, and CP = 0, CP.A=60 J/molK, H RX(TR)= 20,000 J/mol (endothermic), & KC=10 exp[2504T-7.2] p,A A0 A,EB RX R H (T ) C K 1 = X C T T Ae ( ) = X H T 1 1 T 1 RX R R + exp 1 ( ) T T 2 2 1 0.75 0.5 XAe 0.2 when T0 = 600K 0.25 XA 0 200 300 400 500 600 Tadiabatic (T0=600K) XAe 0 at T0 = 298KTadiabatic -0.25 -0.5 -0.75 Equilibrium conversion -1 T (K) Yes, higher conversion is achieved Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.