Practice Problems on Wavelength, Frequency, and Energy
Solve problems relating to wavelength, frequency, and energy of electromagnetic waves. Calculate frequencies, wavelengths, and energies of light in various spectra including violet light and microwave radiation. Understand the relationship between wavelength, frequency, and energy in different scenarios involving hydrogen lamp emissions and sodium bright-line spectra.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
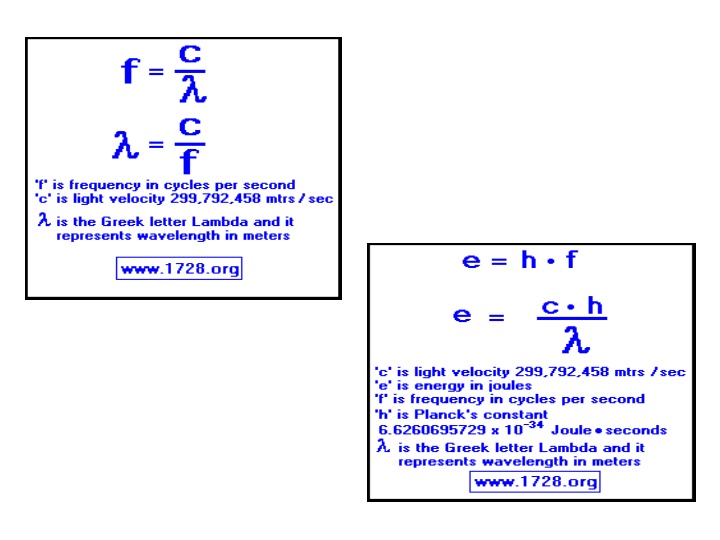
Wavelength, Frequency, and Energy Practice Problems
1. A certain violet light has a wavelength of 413 nm. What is the frequency of this light?
2. What is the wavelength of radiation whose frequency is 1.50 x 1013Hz?
3. How much energy is associated with a photon in the microwave region of the spectrum with a frequency of 6.6 x 1012Hz?
4. A hydrogen lamp emits several lines in the visible region of the spectrum. One of these lines has a wavelength of 6.56 x 10-5cm. What is the energy of this radiation?
5. A very bright line in the bright-line spectrum of sodium has a wavelength of 590 nm. What is the speed of this wave?