Permutations and Combinations in Mathematical Statistics
Permutations and combinations in mathematics, focusing on arrangements and groups formation of distinguishable objects. Learn the concepts symbolically and understand the difference between permutations and combinations for n distinguishable objects taken r at a time.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
M S P Mandals Deogiri College, Aurangabad Department of physics Class :- B.Sc. S. Y.(sem:-lll) course:-Mathematical Statistical physics and Relativity chapter:- statistical basis and classical statistics Topic:-Permutations and Combinations Mrs. S.B.Patil
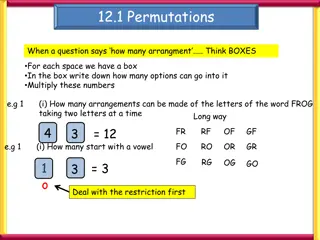
Permutations and Combinations permutations: arrangement and combination means formation of groups. To understand the meaning of permutation i.e. arrangement, let us consider an example of four distinguishable objects marked a, b, c and d. Taking any two objects at a time, the possible arrangements are ab, ba, ac, ca, ad, da, bc, cb, bd, db, cd, dc. The word permutation means
There are total 12 arrangements possible. In arranging these objects the order of their placing is also taken into consideration. Thus, ab and ba are two different permutations. Thus, 4 objects can be arranged in 12 ways by taking 2 objects at a time, i.e. the number of permutations is 12. Symbolically, 4p2= 12
Instead, if 3 objects are taken at a time out of 4 objects a, b, c and d the various arrangements are abc abd acd bcd acb adb ade bdc bca bad cda cbd bac bda cad cdb cab dab dac dbc cba dba dca dcb Thus, 4 objects can be arranged in 24 different ways by taking 3 at a time out of 4 objects. Symbolically, 4p3= 24
In general, the number of arrangements of n distinguishable objects by taking r at a time is given by npr = n /(n-r) .......(1) 4p2 = 4 /(4-2) = 4 /2 = 4x3x2x1 / 2x1 = 12 4p3 = 4 /(4-3) = 4 /1 = 4x3x2x1 / 1 = 24 This includes all types of arrangements, meaningful as well as meaningless. Combinations: Here we consider only combinations i.e. groups without considering the order of their placement i.e. only meaningful combinations.
Thus, the combinations of 4 objects a, b, c and d taking two at a time are ab, ac, ad, bc, bd, cd i.e. only 6 combinations. Symbolically, 4C2 = 6 . Similarly, combinations of 4 objects a, b, c and d taking three at a time are abc, abd, acd, bcd i.e. only 4 combinations. Symbolically, 4C3 = 4
In general, the number of groups i.e. meaningful combinations of n distinguishable object by taking r at a time is given by nCr= n!/r!(n-r)! .........(2) npr = n /(n-r) nCr=nPr/r! nPr = r! nCr from equation 1 npn = n /(n-n) = n / 0 = n (0 =1) from equation 2 nCn= n!/n!(n-n)! = n!/n!(0)! = 1
Macrostate and Microstate Macrostate: Consider 4 distinguishable particles and we wish to distribute them into two exactly similar compartments in an open box. Let the particles be a, b, c and d. When any particle thrown into the box, it must fall into one of the two compartments. Since the compartments are alike, the particles have the same a priori probability of going into either of them and will be . The possible ways in which 4 particles can be distributed in two compartments as shown in table NO. of particles Compartme nt Distributio n 1 0 Distributio n 2 1 Distributio n 3 2 Distributio n4 3 Distributio n 5 4 1 2 4 3 2 1 0
Thus, there are 5 different distribution (0, 4), (1, 3), (2, 2), (3, 1) and (4, 0). Each compartmentwise distribution of system of particles is known as macrostate In general, for a system of n particles to be distributed in two similar compartment, the various macrostates are (0, n), (1, n -1),(2, n- 2),...... (n-1,1) and (n, 0) Therefore, the total no. of macrostates n particles is (n+1).
Macrost ate Possible arrangemtents Compartment 1 No. Of microstates W (Thermodynami cs probability) 1 Compartment 2 Microstates: Since the particles distinguishable, number of different possible arrangements in each compartment is shown below are the 0,4 0 a abcd bcd cda dab abc 4 1,3 b c d 2,2 ab cd bd bc ad ac ab a b c d 0 ac ad bc bd cd bcd cda dab abc abcd 6 3,1 4 4,0 1
Each distinct arrangement is known as the microstate of the system. The distribution (0, 4) can have only one arrangement, distribution (1, 3) can have 4 distinct arrangements, distribution (2, 2) can have 6, distribution (3, 1) have 4 and distribution (4, 0) have only one. Thus, a given macrostate may consists of a number of microstates. In the above example of 4 particles, the total number of microstates are 16 = 2 . In general, for a system of n particles, the total number of microstates are 2 .
Suppose we consider a thermodynamic system of N identical, non-interacting particles occupying a volume V. Let E ,E , E , .......... E be the energy of particles n ,n ,n ......... n respectively, then total energy of the system, E = n E +n E + .......+n E = n E and N= n According to quantum theory, the possible values of energy of each particle are discrete and hence the total energy E also has a discrete value. But when the volume V is very large, the spacings of different energy levels are so small in comparison with total energy of the system that E may be regarded as almost continuous.
The actual values of parameters N, V and E defines a particular macrostate (N, V, E) of the given system. There are a large number of different ways in which the individual Ei s can be chosen, so as to Make total energy equal to E. Each of the different ways in which the total energy E of the system can be distributed among N Particles constituting it specifies a microstate. Thus, a given macrostate corresponds to many microstates and it is very natural to assume that at any time the system is equally likely to be in any of these microstate