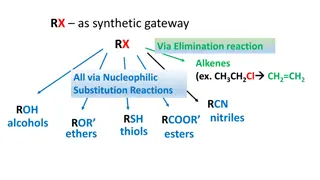
NUCLEOPHILIC ADDITION REACTION
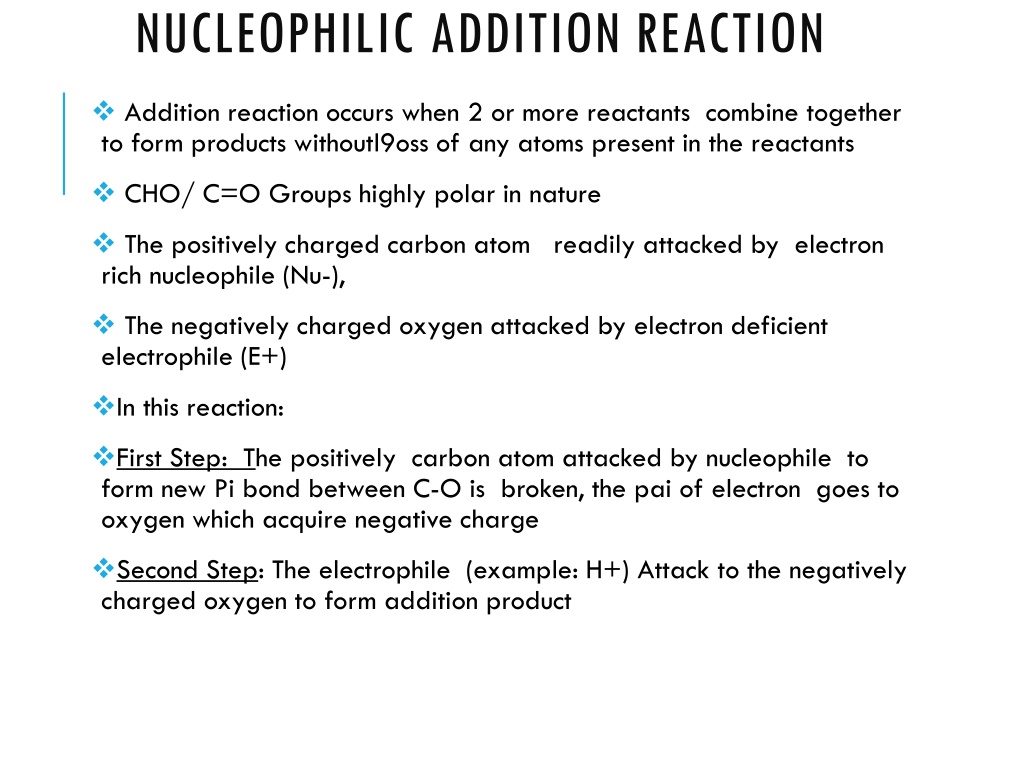
Nucleophilic addition reactions involve the combination of two or more reactants resulting in the formation of products without loss of atoms. This reaction is guided by the highly polar nature of CHO/C=O groups. The mechanism consists of steps where nucleophiles attack positively charged carbon atoms while electrophiles target negatively charged oxygens. Additionally, the role of base and acid catalysis in such reactions is discussed, along with the concept of electromeric effect in influencing organic compound reactions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
NUCLEOPHILIC ADDITION REACTION Addition reaction occurs when 2 or more reactants combine together to form products withoutl9oss of any atoms present in the reactants CHO/ C=O Groups highly polar in nature The positively charged carbon atom readily attacked by electron rich nucleophile (Nu-), The negatively charged oxygen attacked by electron deficient electrophile (E+) In this reaction: First Step: The positively carbon atom attacked by nucleophile to form new Pi bond between C-O is broken, the pai of electron goes to oxygen which acquire negative charge Second Step: The electrophile (example: H+) Attack to the negatively charged oxygen to form addition product
BASE/ ACID CATALYSED ADDITION REACTION 1. Base catalyzed: The Base convert a weak nucleophile to a strong one by removing a proton (H+) The strong nucleophile then add the carbonyl group Nu-H + B ------------NU:- + BH+ (weak neutral) (anionic-strong) 2. Acid catalyzed : Hydrogen from the acid attack negatively charged carbonyl oxygen to give protonated carbonyl group. This is resonance stabilized (NOTE) -Addition product is same in both the cases; - C=O Group is less reactive than -CHO
ELECTROMERIC EFFECT Electromeric effect are electronic factors that influence chemical reactions of organic compounds. Electromeric effect is the complete transfer of pi electrons in a molecule in the presence of an attacking agent. Electromeric effect can be observed in Pi bonds Electromeric effect occurs when a molecule having multiple bonds is exposed to an attacking agent such as a proton (H+). Types: