New Health Information Sharing Requirements: Key Updates
Stay informed about the latest health information sharing requirements effective from April 5, 2021. Learn about the provisions of the 21st Century Cures Act and the obligations for sharing electronic health information. Find out about US Core Data for Interoperability version 1 (USCDI v1) and the exceptions to information blocking. Access helpful resources to ensure compliance and address concerns regarding lab results, clinical documentation practices, and sensitive information documentation.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
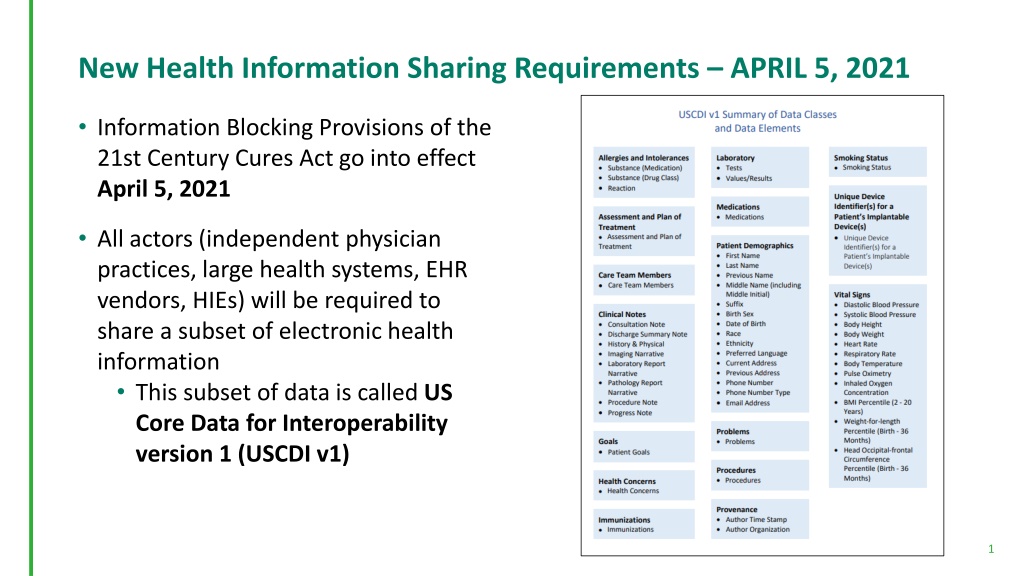
New Health Information Sharing Requirements APRIL 5, 2021 Information Blocking Provisions of the 21st Century Cures Act go into effect April 5, 2021 All actors (independent physician practices, large health systems, EHR vendors, HIEs) will be required to share a subset of electronic health information This subset of data is called US Core Data for Interoperability version 1 (USCDI v1) 1
New Health Information Sharing Requirements APRIL 5, 2021 Required to share unless one of the 8 info blocking exceptions apply E.g., promoting privacy, preventing harm, infeasibility, etc. Concerns We re Hearing: Embargos on lab results (cannot establish "blanket" hold on lab results until physician reviews, could be considered info blocking) Nuance around clinical documentation practices and where to document sensitive information How do you appropriately document to meet one of the 8 info blocking exceptions Questions? Email Brooke at brockwern@acponline.org 2
ACP Interoperability and Info Blocking Resources: www.acponline.org/infoblocking 3
CHIME hosted Info Blocking Resource Center: https://infoblockingcenter.org/ 4