Nature of Subatomic Particles and Light
Explore the intricate world of subatomic particles such as electrons, protons, and neutrons, and delve into the dual nature of light as both particles and waves. Discover the structure of atoms, their isotopes, atomic number, mass number, and the fundamental discoveries in the field of physics, including the wave nature of electrons and light, the photoelectric effect, and the dual behavior of light. Unveil the fascinating history behind the findings through cathode ray experiments, Thomson's charge-to-mass ratio experiment, Millikan's oil drop method, and Rutherford's model with its drawbacks, leading to a deeper understanding of the building blocks of the universe.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
STRUCTURE OF ATOM SUB ATOMIC PARTICLES
DISCOVERY OF SUB ATOMIC PARTICLES CATHODE RAY EXPERIMENTS AND ITS FINDINGS, CHARGE TO MASS RATIO OF ELECTRON- THOMSON S EXPERIMENT, CHARGE ON THE ELECTRONS ON THE BASIS OF MILLIKAN S OIL DROP METHOD, DISCOVERY OF PROTONS AND NEUTRONS
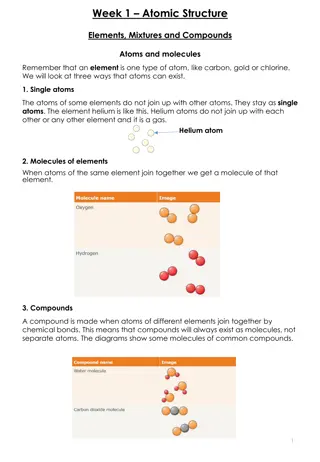
ATOMIC NUMBER AND MASS NUMBER INTRODUCTION - It is equal to the no. of protons present in the nucleus of atoms. It is also equal to the no. of electrons in an atom. ISOTOPES AND ISOBARS - The atoms with same atomic no. but different mass no. are known as isotopes, whereas the reverse is true for isobar. RUTHERFORD S MODEL AND ITS DRAWBACKS- Scattering experiment done with gold leaf with thickness 100 nm Bombarding with fast moving alpha particles and discovery of nucleus o atom. Drawbacks could not explain the stabilioty of an atom .
Wave nature of electron . Maxwell suggested that when electrically charged particle moves under acceleration, alternating electrical and magnetic fields are produced and transmitted. These fields are transmitted in the forms of waves called electromagnetic waves or electromagnetic radiation.
Wave nature of light Light is the form of radiation known from early days and speculation about its nature dates back to remote ancient times. In earlier days (Newton) light was supposed to be made of particles (corpuscules).
Particle nature of light (i) the nature of emission of radiation from hot bodies (black -body radiation) (ii) ejection of electrons from metal surface when radiation strikes it (photoelectric effect) (iii) variation of heat capacity of solids as a function of temperature (iv) line spectra of atoms with special reference to hydrogen.
PHOTO ELECTRIC EFFECT In 1887, H. Hertz performed a very interesting experiment in which electrons (or electric current) were ejected when certain metals (for example potassium, rubidium, caesium etc.) were exposed to a beam of light as shown in The phenomenon is called Photo electric effect.
DUAL BEHAVIOUR OF LIGHT LIGHT possesses both particle and wave-like properties, i.e., light has dual behaviour. Depending on the experiment, we find that light behaves either as a wave or as a stream of particles. Whenever radiation interacts with matter, it displays particle like properties in contrast to the wavelike properties (interference and diffraction), which it
HYDROGEN SPECTRUM The hydrogen spectrum consists of several series of lines named after their discoverers. Balmer showed in 1885 on the basis of experimental observations that if spectral lines are expressed in terms of wavenumber (n ).
De Broglie equation as the photon has momentum as well as wavelength, electrons should also have momentum as well as wavelength, de Broglie, from this analogy, gave the following relation between wavelength (l) and momentum (p) of a material particle.
HEIGENBERGS UNCERTAINTY PRINCIPLE It states that it is impossible to determine the exact position and momentum of electron simultaneously.
QUANTUM NUMBERS It determines the complete address (identity ) of an electron . Principle Quantum Number , Azimuthal Quantum Number, Magnetic Quantum Number Spin Quantum Number
Shapes of Atomic Orbitals s orbital is spherical, p orbital is dumb-bell shaped, d orbital is double dumb-bell shaped, f orbital is highly complex.
Paulis Exclusion Principle No two electrons in an atom can have the same set of four quantum numbers . Only two electrons may exist in the same orbital and these electrons must have opposite spin.
Hunds Rule of Maximum Multiplicity Pairing of electrons in the orbitals belonging to the same subshell (p,d,f) does not take place until each orbital belonging to that subshell has got one electron each. Half- filled or fully- filled orbitals are more stable than the incompletely filled orbitals. Reason for this is its symmetry and exchange energy .
Aufbau Principle The lower the value of (n+l) for an orbital, the lower is its energy . If two orbitals have the same value of (n+l) , the orbital with lower value of n will have the lower energy and in the ground state of the Atoms, the orbitals are filled in order of their increasing energies : 1s, 2s, 2p , 3s , 3p , 4s, 3d, 4p, 5s,4d, --