Mechanistic Analysis in Organic Chemistry
The concept of chemical mechanisms in the context of organic chemistry is explored, focusing on hypothesis-driven research, mechanistic questions, and the workflow for mechanistic analysis. Understanding and formulating valid mechanistic hypotheses is essential for predicting and explaining chemical outcomes. The importance of control experiments in identifying the roles of reaction components is emphasized.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
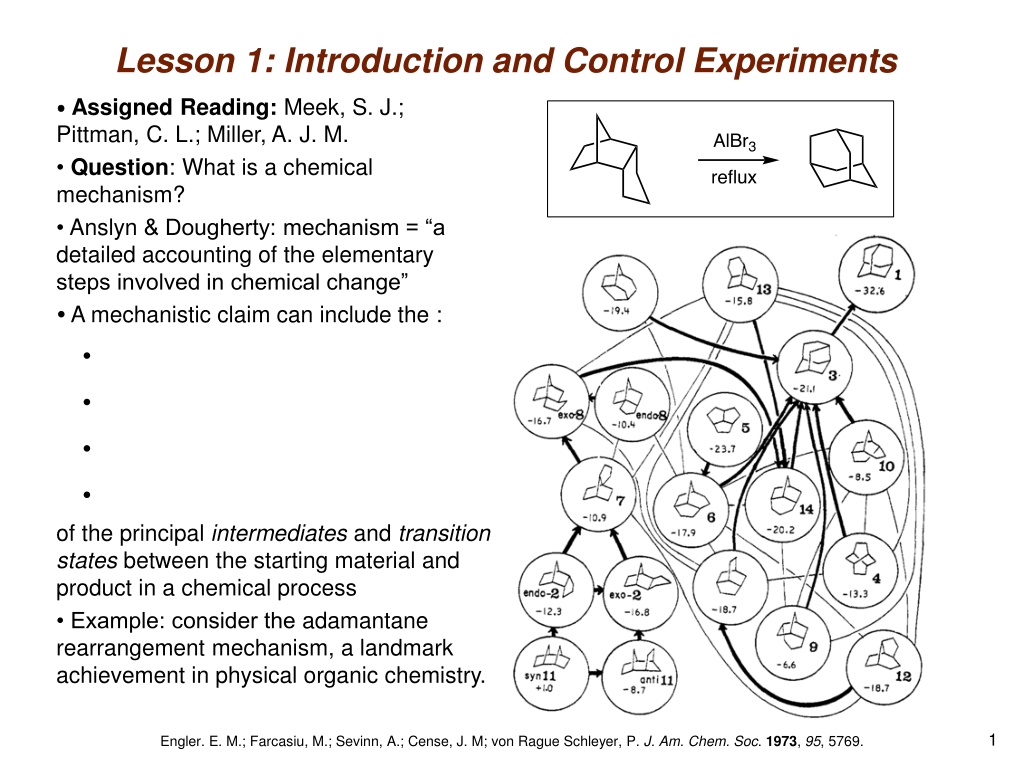
Lesson 1: Introduction and Control Experiments Assigned Reading: Meek, S. J.; Pittman, C. L.; Miller, A. J. M. Question: What is a chemical mechanism? Anslyn & Dougherty: mechanism = a detailed accounting of the elementary steps involved in chemical change A mechanistic claim can include the : of the principal intermediates and transition states between the starting material and product in a chemical process Example: consider the adamantane rearrangement mechanism, a landmark achievement in physical organic chemistry. 1 Engler. E. M.; Farcasiu, M.; Sevinn, A.; Cense, J. M; von Rague Schleyer, P. J. Am. Chem. Soc. 1973, 95, 5769.
Hypothesis-Driven Mechanistic Analysis Most chemical endeavors do not allow or require this level of mechanistic detail. The standards for a satisfactory mechanism depend on the goals of the study. What are some of the goals one might have to study mechanism in organic chemistry? The study of mechanisms represents hypothesis-driven research: A mechanistic hypothesis: It must be possible to make predictions about chemical outcomes based on a mechanistic hypothesis or the hypothesis is not useful for the goals above Experiments that test the predictions of a hypothesis are called hypothesis testing. Mechanistic studies usually rely on negative evidence: a mechanistic hypothesis is never proven but it can be accepted as more plausible as the alternatives are shown to be incompatible with chemical outcomes obtained by hypothesis testing. Positive mechanistic evidence (in situ spectroscopy, modeling) are not covered in this course 2
Mechanistic Questions Mechanistic hypotheses must be falsifiable by empirical evidence or else they cannot be evaluated through hypothesis testing. When considering multiple mechanisms, the point at which they disagree is a mechanistic question. A valid mechanistic question must have: Multiple plausible mechanistic hypotheses as alternatives. An implication for prediction of future reactions Example 1: Is there a valid mechanistic question here? + + NaCl Hypothesis 1: + NaCl Hypothesis 2: + NaCl 3
Mechanistic Questions Example 2: Is there a valid mechanistic question here? 4
Mechanistic Questions Example 3: Is there a valid mechanistic question here? AcOH + racemic 100% ee Hypothesis 2: Hypothesis 1: TfO TfO 5
Workflow for Mechanistic Analysis Isolate and characterize the reaction components, conditions, and products (specify the reaction and its outcome) Generate valid mechanistic hypotheses that explain the product outcome within accepted paradigms of organic chemistry Control Experiments identify which component (reactant, reagent, or condition) serves which role in the reaction Formulate and test predictions about reaction outcomes for each hypothesis, and test them with a mechanistic experiment. Based on outcome, draw conclusions, reformulate your hypotheses or design new mechanistic experiments. 6
Outline of this Course First step: Describing the chemical reaction: what was put into the reaction, what was done to the reaction, and what products were obtained + ligand I am taking this for granted, but you will lose points if you leave out essential information here. Control experiments (this lesson): The first lesson teaches how to identify which component participates in the reaction and to what capacity by probing them individually. Mechanistic hypotheses (lessons 2-4): The second section of this course covers the relevant paradigms of organic reaction mechanisms, which can be combined with topic- specific literature searching in order to propose a mechanism that would make sense to organic chemists. Mechanistic experiments (lesson 1b and lessons 5-7): A variety of physical techniques rely on how subtle variations in the reagents or conditions effects the rate or outcome of a chemical reaction as a way to answer mechanistic questions. Conclusions (Lessons 8 & 9): the last section covers physical chemistry topics that further help to interpret data from mechanistic studies. 7
Control Experiments In general, a control experiment distinguishes the role of a single macroscopic variable in a complex system. In a mechanistic study these variables include: Even when considering one variable, multiple control experiments are often necessary because they give complementary information. Consider an analogy (medicine): Sick patients at a hospital are given a new, experimental medicine and then most of them get better. Mechanistic question: Does the active pharmaceutical ingredient (API) cause the patients to get better? The API is the single, macroscopic variable of interest Hypothesis 1: Hypothesis 2: Hypothesis 3: Control experiment: at least two experiments will need to be performed here Experiment 1: Experiment 2: 8
Control Experiments What are the predicted outcomes for each hypothesis? Hypothesis 1: Hypothesis 2: Hypothesis 3: What if you had only run one of the two experiments? In what way can the outcome be inconclusive? The most common chemistry control experiments fall into four categories: 1. Omission experiments (leave it out, e.g. no pill) 2. Scavenging experiments (take it out) 3. Replacement experiments (swap it out, e.g. the placebo) 4. Resubmission experiments (put it back in) 5. Validity experiments (show that your experimental setup works) 9
Omission Experiments Omission experiments ask the most basic mechanistic question: is this component a spectator or an essential participant in the reaction? If the component is a spectator, then leaving it out should not affect the reaction. If the component is essential, then omission should result in no reaction However, there is always a third possibility: the component plays a role but it is not essential. In that case, omission results in a change (in rate or yield etc.) but not a total end to the reaction. This case is common for catalysts Example: palladium catalysis: 5 mol% + 80% yield Pd(PPh3)4 omission experiment: everythingin this experiment is the same except the Pd(PPh3)4 has been left out. Outcome??? + 10
Omission Experiments There are generally three possible outcomes of the Pd(PPh3)4 omission experiment: Outcome 1: Outcome 2: Outcome 3: Caveat: If any of the components is contaminated with palladium then you are not really omitting palladium in this experiment Reactivity observed in absence of a catalyst is called background reaction. Example 2: Special conditions (beyond mix + heat) usually call for omission controls Special conditions: Specific example: CH3CN, H2O 65% yield NaHCO3 h ~ 250 nm What would UV omission reveal? What other control experiments are necessary? 11
Scavenging Experiments In a scavenging experiment, the role of a byproduct or intermediate that was not put into the flask is evaluated by selectively removing it from the system. Typically a scavenger is added to selectively react with that component. There is an assumption here. Example 1: Mercury drop test: liquid Hg is known to scavenge metal nanoparticles Suppose in the following reaction you have established that rhodium is essential (how) but you want to show that the reaction does not involve Rh(0) nanoparticles: RhCl(PPh3)3 95% yield + H2 CH2Cl2 Perform the reaction again with a drop of mercury. The possible outcomes: Outcome 1: Outcome 2: Outcome 3: 12
Scavenging Experiments Example 2: Hot filtration: the reaction is filtered while the reaction is still occurring (e.g. hot ) to remove any solids Usually, this approach tests a claim of heterogeneous catalysis, but it can also test the involvement of a precipitate formed under reaction conditions. 5 mol% Pd/C + 80% yield Filter the reaction before it is done. The possible outcomes: Outcome 1: Outcome 2: Outcome 3: 13
Scavenging Experiments Example 3: Molecular sieves: irreversibly absorb water from a reaction 5 mol% catalyst + catalyst CH2Cl2 80% yield, 92% ee Adding molecular sieves to this reaction resulted in a considerable drop in yield. The explanation is that water is responsible for amine catalysis: + + H2O Radical scavengers: if addition of a radical scavenger stops a reaction then you can conclude that a radical component (usually intermediate) is essential R R radical acceptor TEMPO stable radical But, TEMPO can be oxidized and reduced or react in other ways and so it may not behave as a pure radical scavenger. 14
Replacement Experiments Like the placebo experiment in the medicine example, replacement tests whether an alternative reagent or condition has the same effect. Replacement experiments are used to complement omission/scavenging experiments. Example 1: photochemistry CH3CN, H2O 65% yield NaHCO3 h ~ 250 nm Omission of UV (dark reaction) results in 0% yield. But before concluding that the reaction is photochemical you would also have to: Replacement 1: Replacement 2: 15
Replacement Experiments Example 2: acid catalysis 20 mol% Bi(OTf)3 80% yield + CH2Cl2 Background: omission of Bi(OTf)3results in 0% yield, but that doesn t mean that the active catalyst involves Bi. Instead, hydrolysis could lead to TfOH, which would not be formed in the absence of Bi(OTf)3. Replacement experiment: 20 mol% TfOH 80% yield + CH2Cl2 Outcome 1: Outcome 2: Outcome 3: Alternative: replacement can be done progressively, e.g. 10 mol% Bi(OTf)3 + 10 mol% TfOH. 16
Resubmission Experiments In a resubmission experiment, you are observing what happens to a compound and what influence that it has on the reaction after you add it to the reaction. Product resubmission: asks the mechanistic question is the product once formed, a spectator or does it continue to react or influence the reaction? Intermediate resubmission: asks the mechanistic question is this substance a plausible intermediate in this reaction? Labeling: if you want to know what happens to the resubmitted material, you should label it somehow to distinguish it from the other products of the reaction. Assumption: labeling should have a limited influence on reactivity Example 1: Product inhibition is defined as a kinetic situation where the product, once formed, reduces the rate or yield of the subsequent reaction. This is possible, for example, when the product can coordinate to a metal catalyst. Pd(PPh3)4 65% yield + KF, dioxane Question: Does the product inhibit the reaction? 17
Product Resubmission Control: resubmit a labeled version of the product. + Pd(PPh3)4 + KF, dioxane + In analyzing the outcome, you should look for two pieces of information: 1. How did the yield of the unlabeled product change? Outcome 1: Outcome 2: Outcome 3: 2. What happened to the resubmitted, labeled material? Outcome 1: Outcome 2: 18
Product Resubmission Often, the product of a reaction is not totally stable under the conditions that it is formed Product resubmission and recovery allows you to determine what happens to the product under the reaction conditions. The primary data would be recovered yield or recovered stereochemistry. CAN 75% yield 95% ee TFA + + acetone 20 mol% Does the product decompose or racemize under these conditions? Product resubmission: 74% yield 95% ee TFA + + CAN 20 mol% acetone 90% recovery 80% ee + 95% ee 19
Product Resubmission Reversibility: do the products and starting materials interconvert? or 2 + Resubmission: add a labeled product. If exchange or crossover occurs, then you can attribute it to reversibility. + 2 + expected if not reversible + crossover product implicates reversibility 20
Intermediate Resubmission Intermediate resubmission tests whether the specified substance could be an intermediate, based on whether it gets converted to the desired product under the reaction conditions. Again, resubmission does not conclusively prove that it is an intermediate just because it gets converted to the desired product However, resubmission can conclusively prove that something is not an intermediate if it does not react under the reaction conditions. Resubmission is only possible if the proposed intermediate can be isolated. Example: Petasis reaction. + CH2O + PhB(OH)2 80% yield dioxane Test whether a hemiaminal is an intermediate by preparing and resubmitting one + CH2O + PhB(OH)2 80% yield dioxane or + 21