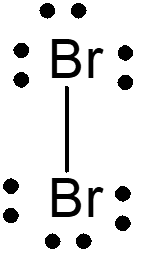
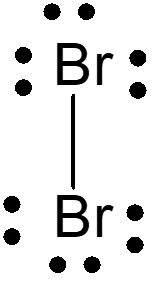
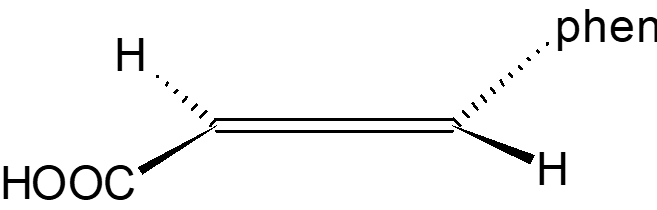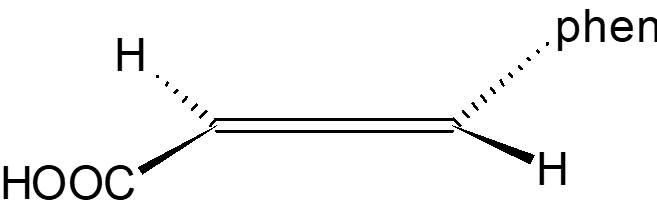Mechanism of Br2 Attack on Trans-Cinnamic Acid
The detailed anti-attack and syn-attack mechanisms of Br2 on trans-cinnamic acid are presented, illustrating the intricate steps involved in preventing or facilitating the attack of bromine on the compound. The process involves initial attack, bridgehead formation, bond rupture, bond stabilization, and final structural reconfigurations. Through the insightful images and descriptions, the molecular dance of electrons and bonds unfolds, guiding us through the protective and reactive strategies at play in the chemical realm.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Topside, anti-attack mechanism for Br2 on trans-cinnamic acid I m on top of things !
1) Initial head-down Br2 attack 2) Initial bridgehead formation Electrons make incipient bridge head bond with Br Br-Br bond begins to break bond begins to break Br - Br Br Br phen phen + H H H HOOC H HOOC
3) Br-Br bond rupture and deformation/near dissolution of bridgehead (favoring carbocation on the electron rich `phenyl=phen side) (-) A very weak linkage exists here until Br(-) attacks, preventing rotation of phen and H Br- Br Br (+) phen + phen H H H HOOC H HOOC Br-
4)Final Anti attack of Br(-) at (+) side where phenyl=phen stabilizes the intermediate carbocation phen Br Br H + phen H H HOOC H HOOC Br Br-
5a) Rotate 3D projection to Fischer eclipsed form Eclipsing end group (Rotated into plane) phen Br H H H CW Br Br H HOOC Br CW phen COOH Eclipsing end group (rotated into plane)
5B) draw 3D double chiral center form in vertical format 5C) convert to 2D final form Br H H COOH H Br R Br Br H COOH Br H Br H phen COOH S phen phen
Topside, syn-attack mechanism for Br2 on trans-cinnamic acid I prefer lying on my side !
a) Single electron transfers from Br lone pairs and from system to form two incipient bonds to carbons b) system and bond between two Br starts to dissolve. c) COOH, H and H,phen groups flex downwards away from attacking Br as bonds form from Br to both sides of trans-cinnamic acid 1) Initial parallel Br2 attack Br Br Br Br phen H phen H H HOOC HOOC H d) Br-Br bond disappears and bond disappears
Once the parallel attack is complete , the two Br eclipse each other , and both the Br-Br bond and the bond are gone As with the anti attack, the structure is then rotated to eclipse the phen and COOH Br Br Br H Br H phen H H HOOC COOH phen
The 3D eclipsed form is turned so that the projection is vertical: COOH Br H Br R H Br H Br H COOH H Br H COOH phen Br R phen phen Finally, the 3D vertical form is converted to the 2D Fischer projection