Key Determinants of PFOA Half-Life and Renal Resorption
Uncover insights into species and gender differences impacting PFOA half-life and renal resorption. Explore how saturation affects clearance and nonlinearity at high doses. Dive into MCMC analysis of the Elcombe Clinical Study, shedding light on PFOA mean half-life in humans. Recommendations for further studies and understanding interspecies pharmacokinetic adjustments for PFOA are provided. Delve into the challenges of risk assessments for PFOA, including the significance of Dose-Dependent Transition Associated with Activation of PPAR Exposures.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
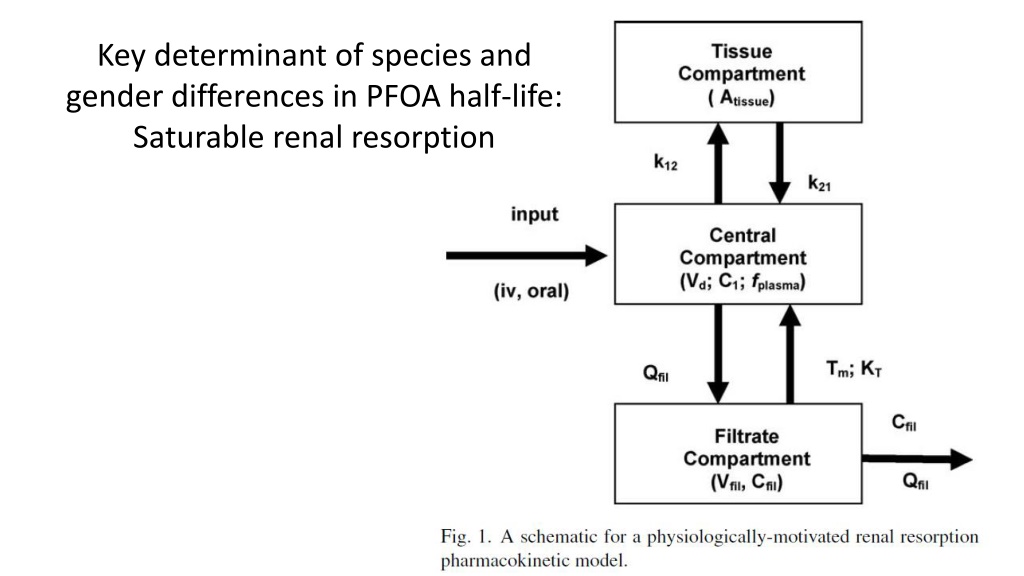
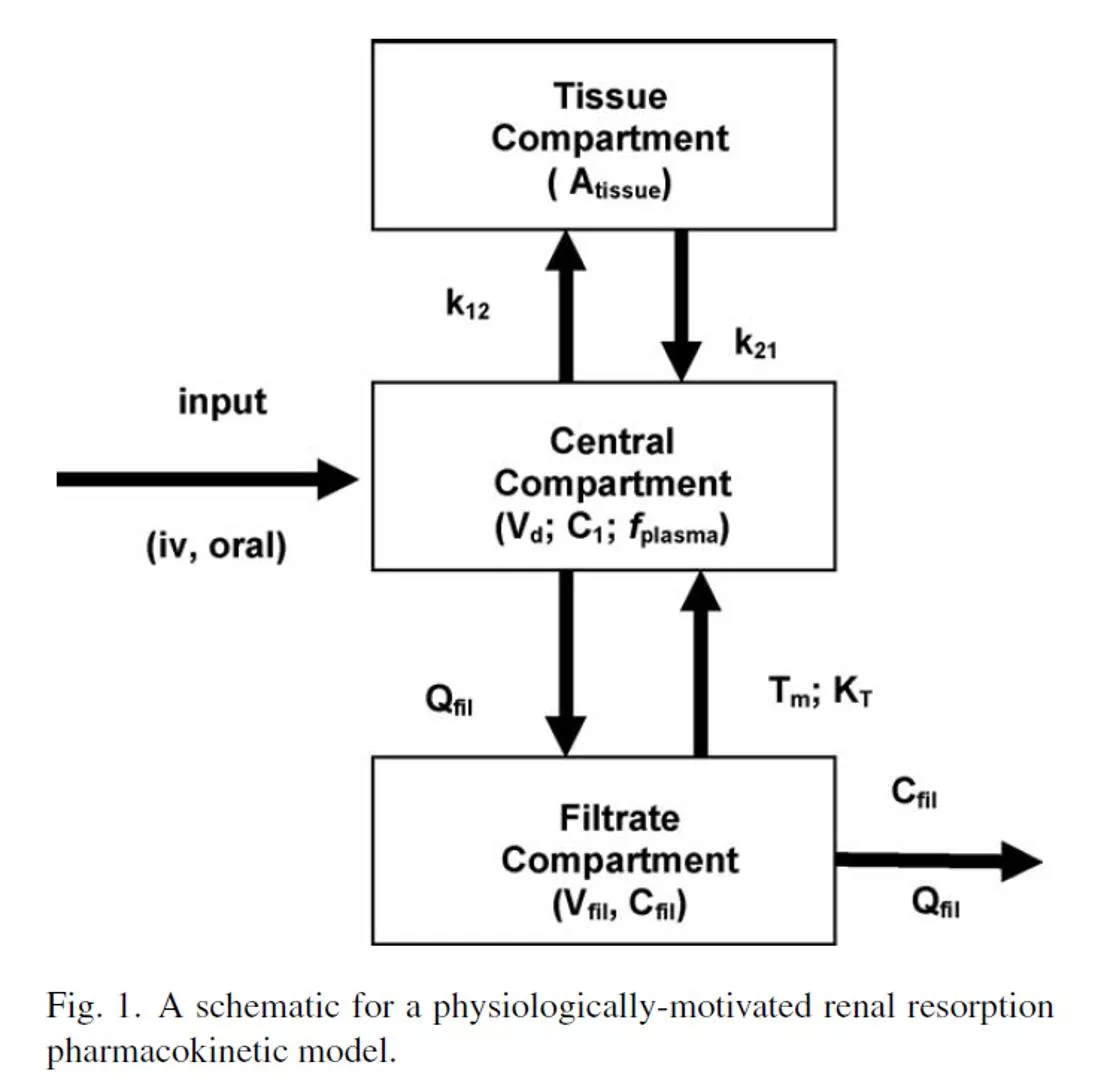
Key determinant of species and gender differences in PFOA half-life: Saturable renal resorption
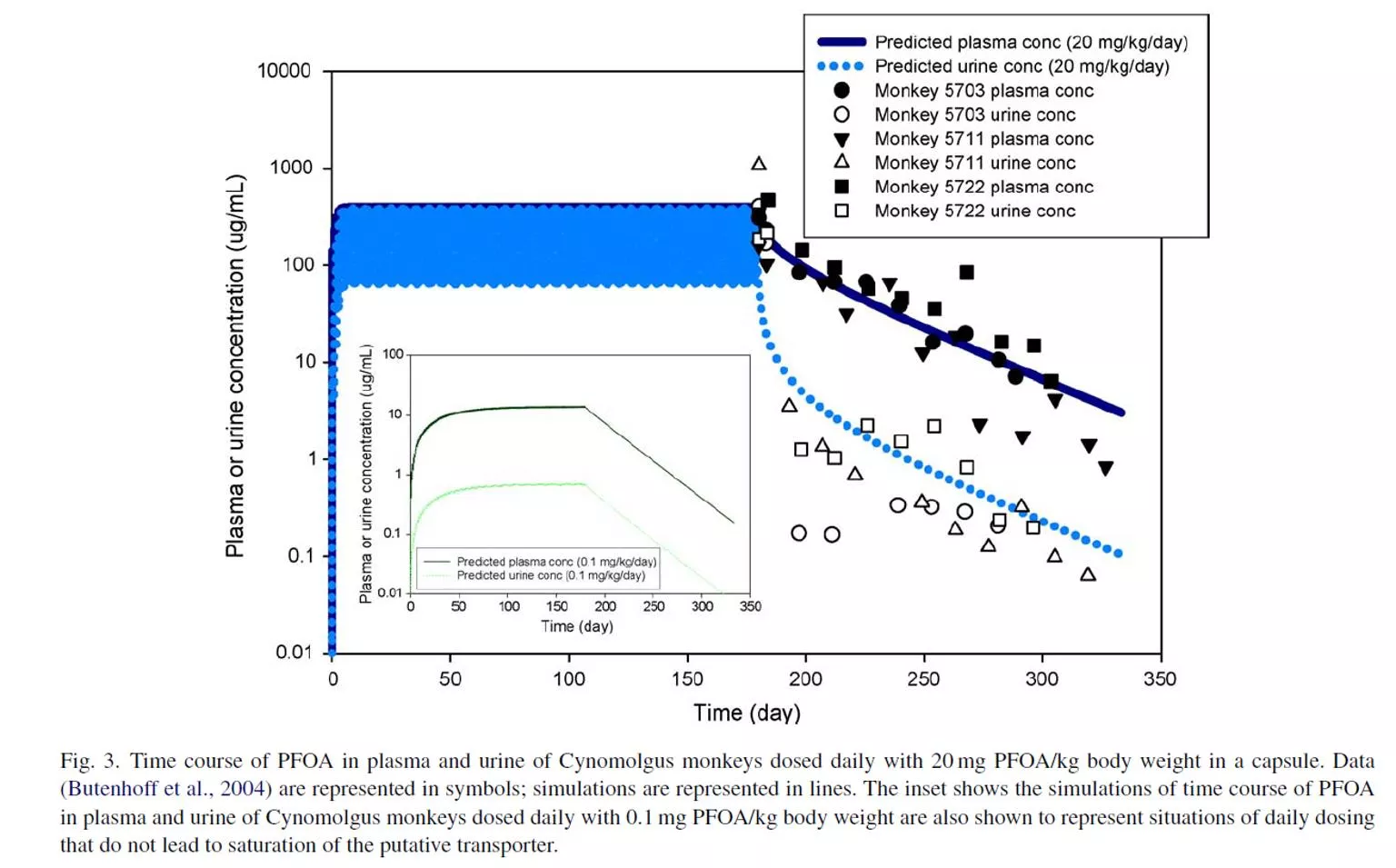
Saturation of resorption leads to more rapid clearance at high doses
Saturation of resorption leads to nonlinearity at high doses
Preliminary MCMC Analysis of Elcombe Clinical Study (Campbell et al. 2016)
Final MCMC Analysis of Elcombe Clinical Study PFOA Mean t1/2 = 1.8 yrs. (Campbell et al. 2021, in preparation)
Recommendation The CXR database provided a unique opportunity to incorporate a controlled human study in the calibration of the human PFOA PBPK model. The clinical data were most useful in establishing the maximum rate and affinity constants for saturable resorption in the kidney which determine the half-life in humans. Nevertheless, there is residual uncertainty in the half-life of PFOA in humans, as most of the participants in the CXR study were still above saturation of renal resorption upon termination of sampling. Additional data from controlled exposures at lower doses would complement this study. PFOA blood concentrations should be determined in each subject prior to dosing.
A DDEF for PFOA In the rodent, the half life of PFOA is on the order of 1-5 days and the duration of dosing in the developmental studies is long enough to reach pseudo-steady state The human half life is on the order of 1.8 years and the exposures of concern are chronic (multigenerational), so they are at pseudo-steady state Therefore, even in the case of developmental toxicity, the ratio of the daily doses that will result in the same blood concentration in the animal and human is the inverse of the ratio of the urinary clearance, which in turn is inversely related to the half-life (ke=ln2/t1/2) Thus, the interspecies pharmacokinetic adjustment is the just ratio of the half-lives For PFOA, EFAK 131 (657/5) to 657
The real problem with risk assessments for PFOA: Failure to recognize a Dose-Dependent Transition Associated with Activation of PPAR Exposures Effects Andersen et al. 2021 (submitted)