Ionic Compound Formulation and Nomenclature
Ionic compounds consist of cations and anions combined in simple ratios to balance charges. The cross technique helps determine the correct ratio of ions. Reduction of ratios may be necessary in certain cases. Formulation and naming examples are provided to illustrate these concepts.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
IONIC COMPOUND FORMULATION & NOMENCLATURE
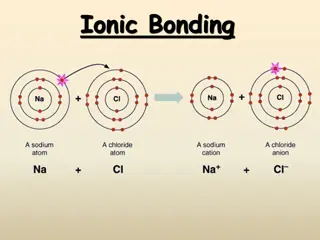
All ionic compounds consist of two parts: a cation (a positive ion) and an anion (a negative ion). Ions are combined in simple, whole-number ratios to balance their charges. This results in a net charge of zero, which all ionic compounds MUST have. The cross technique is used to create the correct ratio of ions needed. Example: aluminum (Al+3) and oxygen (O-2). AlO 1. Write the symbols, then write the charges on each symbol (from ion list) +3 -2 +3 -2 Al O 2 3 3. Clean up the formula (completely erase all charges) and reduce if necessary. AlO 2 3 2. Cross the charges, dropping the + and symbols (don t write 1s) So when do you need to reduce?
Heres two examples of when the ratio needs to be reduced: +2 -2CaO -2SnS CaO SnS 22CaO 24SnS2 2 +4 Let s try formulating some compounds (putting them together):
Lets try formulating some compounds (putting them together): MgF2 FeBr3 KCl CaCl2 Ag3N Mg+2F- Fe+3Br- Ca+2Cl- 6. tin (II) sulfide = ___________________ 7. barium phosphide = _______________ 8. cobalt (III) oxide = _________________ 9. copper (I) iodide = _________________ 10. lead (IV) selenide= ________________ K+Cl- Ag+N-3 1. magnesium fluoride = ______________ 2. iron (III) bromide = ________________ 3. potassium chloride = ______________ 4. calcium chloride = ________________ 5. silver nitride = ____________________ Let s try naming some compounds: 1. FeO ___________________ 6. Mn3P2 ___________________ 2. Al2O3 __________________ 7. Na2S____________________ 3. K2O____________________ 8. Ag2S____________________ 4. PbO2 __________________ 9. Cu3N____________________ 5. K2S ___________________ 10.ZnCl2 ___________________ MONATOMIC
Lets try formulating some compounds (putting them together): MgF2 FeBr3 KCl CaCl2 Ag3N Sn+2S-2 Ba+2P-3 Cu+I- Sn2S2 Ba3P2 Co2O3 CuI Pb2Se4 PbSe2 SnS 6. tin (II) sulfide = ___________________ 7. barium phosphide = _______________ 8. cobalt (III) oxide = _________________ 9. copper (I) iodide = _________________ 10. lead (IV) selenide= ________________ Co+3O-2 Pb+4Se-2 1. magnesium fluoride = ______________ 2. iron (III) bromide = ________________ 3. potassium chloride = ______________ 4. calcium chloride = ________________ 5. silver nitride = ____________________ Let s try naming some compounds: 1. FeO ___________________ 6. Mn3P2 ___________________ 2. Al2O3 __________________ 7. Na2S____________________ 3. K2O____________________ 8. Ag2S____________________ 4. PbO2 __________________ 9. Cu3N____________________ 5. K2S ___________________ 10.ZnCl2 ___________________ MONATOMIC
Ionic compounds are named with the following rules: The cation name goes first, the anion name goes second. cations are usually metals. The name of the cation does not change from the name of the element. Zn+2 = zinc Sc+3 = scandium Fe+2 = iron (II) Fe+3 = iron (III) Note: If the element is polyionic (meaning it can have more than one type of charge), this must be indicated by placing a roman numeral after the name to indicate the size of the charge. anions are a single negatively charged element (monatomic) name of element with ending taken off and -ide added.
Lets try formulating some compounds (putting them together): 1. magnesium fluoride = ______________ 2. iron (III) bromide = ________________ 3. potassium chloride = ______________ 4. calcium chloride = ________________ 5. silver nitride = ____________________ Ag3N Sn2S2 Ba3P2 Co2O3 CuI PbSe2 SnS MgF2 6. tin (II) sulfide = ___________________ 7. barium phosphide = _______________ 8. cobalt (III) oxide = _________________ 9. copper (I) iodide = _________________ 10. lead (IV) selenide= ________________ Pb2Se4 FeBr3 KCl CaCl2 1. FeO ___________________ iron ( ) oxide II manganese ( ) phosphide II Let s try naming some compounds: 2. Al2O3 __________________ aluminum oxide potassium oxide lead ( ) oxide potassium sulfide 6. Mn3P2 ___________________ sodium sulfide silver sulfide copper ( ) nitride zinc chloride 7. Na2S____________________ 3. K2O____________________ IV 8. Ag2S____________________ I 4. PbO2 __________________ 9. Cu3N____________________ 5. K2S ___________________ 10.ZnCl2 ___________________
COVALENT BONDING CHARACTERISTICS AND NOMENCLATURE
Covalent compounds share electrons, (ionic bonds transfer them). Covalent bonds = two nonmetals, or a nonmetal and a metalloid. Remember, hydrogen is not an alkali metal, it is a nonmetal. Remember, ionic bonds always contain a metal. Metals never appear in covalent compounds!!! Covalent bonds rely on the octet rule, meaning that atoms in a covalent bond need 8 electrons around them (in most cases). covalent bonds can be single, double, or triple, in order to satisfy the octet rule. CF4 CO2 F F C F O C O N N F N2 1 triple bond 4 single 2 double bonds Lewis-dot structures show the arrangement of these eight electrons bonds
covalent compounds can involve the same elements with multiple ratios (such as C3H8, C2H4, NO2, N2O), so the ratio in the compound must be shown in the name. to write the name of a covalent compound, we use Greek prefixes: attach the appropriate prefix to the name of each element in the compound. (Note: if the first element in the compound is single, you do not need the prefix mono-. Mono- is only used on the second element!) the ending of the secondelement s name is taken off, and -ide is added. if you get any of the following vowel combinations in the name when you add the prefix: oo, ao, oa, or aa, that s not allowed! Drop the vowel off of the prefix, and then add it to the name. for diatomic gases, like O2, name the element, then add the word gas. Therefore, O2 = oxygen gas # 1 2 3 4 5 6 7 8 9 Prefix mono- di- tri- tetra- penta- hexa- hepta- octa- nona- deca- 10
Lets try a few: C2N6 = _____________________ dicarbon hexanitride triphosphorus heptaoxide dihydrogen monooxide pentaboron monoarsenide nitrogen gas 1. no AO combo! no OO combo! no OA combo! heptoxide monoxide monarsenide (diatomic gas!) 2. P3O7 = _____________________ H2O = ______________________ 3. 4. B5As = ______________________ N2 = _______________________ 5. S3Cl4 6. trisulfur tetrachloride = ___________ CO carbon monoxide = ___________ 7. Cl2(diatomic gas!) 8. chlorine gas = ____________ SiBr5 9. silicon pentabromide = ___________ Cl4Br10 10. tetrachlorine decabromide = ___________
Can you tell the difference between an ionic compound and a covalent compound based on the name or formula alone? You need to be able to do so! Place an I or a C in the blank: 1.Fe3N2 2.K2S 3.NH3 4.Br2 5.Mn2O3 6.CaO 7.Li2O ___ ___ I I C C I C I I C I ___ ___ ___ ___ ___ ___ I I ___ ___ ___ ___ ___ ___ I I 8. carbon dioxide 9. aluminum nitride 10.tin (II) sulfide 11.chlorine gas 12.calcium sulfide 13.cobalt (III) oxide 14.sodium phosphide