Intense Muon Beams for Higgs Studies at ESS SB Project
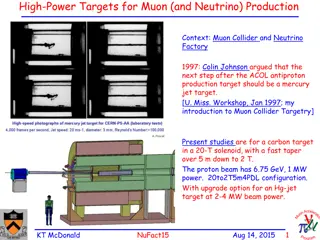
The ESS SB project aims to create an intense muon beam facility for studying the Higgs sector. It involves muon production, cooling, and acceleration to enable Higgs studies. The project includes a high-intensity H-source, compression rings, ionization cooling system, LINAC acceleration, and collider rings. GEANT4 simulations and proton rings are utilized in the production process. The project's technical details and setup are outlined through GEANT4 simulations and proton ring configurations.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
WEL COME Department of Chemistry Department of Chemistry Dr. S.V. Lamture
HETEROCYCLIC COMPOUND A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its rings. Heterocyclic compound are cyclic compounds in which the ring atoms are of carbon and some other element. The atom of the other element (for example: N , S , or O) is called the Hetero atom. For example: Pyrrole, Furan , Thiophene, Indole , pyridine , Quinoline ,etc. Thiiran Pyridine Furan
Three Membered Rings compounds Seven Membered Rings compounds Classification of Heterocyclic compounds according to it s membered Four Membered Rings compounds Six Membered Rings compounds Five Membered Rings compounds
1.PYRROLE(AZOLE) C4H5N The pyrrole ring system is important as it is found in many natural products including hemoglobin, chlorophyll, and alkaloids. According to the resonance theory ,pyrrole is considered to be hybrid of the following five resonance structures.
PREPARATION OF PYRROLE o Pyrrole is obtained : 1. Industrially pyrrole is obtained by passing a mixture of furan and ammonia over alumina at 400 C. Al2O3 Furan Pyrrole 2. Pyrrole can be obtained by heating 2-bytyne-1,4-diol with ammonia under pressure. Pyrrole
PROPERTIES OF PYRROLE Physical properties of Pyrrole discussed below it: 1. It is a colourless volatile liquid. 2. It s boiling point is 131 C and melting point is -23 C. 3. It turns brown in the air and gradually resinifies. 4. Only slightly soluble in water but it is totally miscible with ether and ethanol. 5. Pyrrole is weakly basic in nature. Chemical properties of pyrrole discussed below it: Pyrrole is aromatic and more reactive than benzene. It gives electrophilic substitution reactions such as halogenation , nitration ,etc. It also undergoes diazotization and Reimer-Tiemann reactions, while benzene does not. 1. 2. 3.
USES OF PYRROLE Pyrrole is essential to the production of many different chemicals. N-methylpyrrole is a precursor to N- methylpyrrolecarboxylic acid, a building-block in pharmaceutical chemistry. Although there is a claim that pyrrole is used as an additive to cigarettes, it is typically listed as a constituent of tobacco smoke and not as an ingredient. Pyrrole is also use in commercial and pharmaceuticals.