Exploring Copper Sulfate, Sodium Nitrate, and Ammonium Chloride in Chemistry Projects
Delve into the properties and uses of copper sulfate, sodium nitrate, and ammonium chloride in chemistry projects. Discover how these chemical compounds play vital roles in agriculture, industry, and everyday applications, from fungicides to food additives.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
CHEMISTRY PROJECT By: Lujain Barakat , Yasir Sultan , Laith Hamarneh , Kareem Aldeen and Maya Makhoul
COPPER SULFATE Copper Sulfate is an inorganic, compound with the chemical formula CuSO4. It is a blue solid that can kill fungi. It is used to purify copper metals. Other names for copper sulfate are : cupric sulfate, bluestone and blue vitriol. Solubility is in water is 31.6 g / 100 ml (0 degrees Celsius) The melting point is 150 degrees Celsius (423 K) Dehydrates, 650 degrees Celsius.
HOW DO WE USE COPPER SULFATE? Copper sulfate is used as a fungicide, root killer, and herbicide in both agriculture and non-agriculture settings. It is also used as an antimicrobial. Copper sulfate is used as a drying agent in the anhydrous form, as an additive for fertilizers and foods, and several industrial applications such as textiles, leather, wood, batteries, Ink, petroleum, paint, and metal, among others. It is used also an animal nutritional supplement. Copper sulfate is not a skin irritant.
SODIUM NITRATE Sodium Nitrate is a polyatomic ion with the chemical formula NaNO3. Salts containing this ion are called nitrates. Sodium Nitrate is known for in the production of nitric acid, model rocket propellants and several types of fireworks. Sodium Nitrates are common components fertilizers and explosives. Almost all nitrates are soluble in water.
HOW DO WE USE SODIUM NITRATE Sodium nitrate is mined or synthetically produced and used as fertilizer, a food preserve in hot dogs and sausages, an ingredient in glass and ceramics. It was once of strategic importance because of its use in gunpowder. By dilating the blood vessels of the heart, nitrates can reduce the stress on the heart by improving blood flow to the heart muscle.
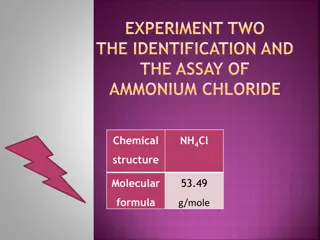
AMMONIUM CHLORIDE Ammonium chloride has the formula NH4Cl and a white crystalline. Ammonium chloride is highly soluble in water. Solutions of ammonium chloride are mildly acidic. It is the product from the reaction of hydrochloric acid and ammonia. It is soluble in water. Ammonium chloride is a systemic and urinary acidifying salt.
HOW DO WE USE AMMONIUM CHLORIDE It is mainly used as fertilizers and flavouring agent in some types of liquorice. Its principal uses are as a nitrogen supply in fertilizers and as an electrolyte. It is used in food additives in bread making as a yeast nutrient. It is used in medicine. Ammonium chloride is used in cooling baths to create low temperatures.
POTASSIUM NITRATE Potassium nitrate is white or colorless. Potassium is the primary agent for common, over the counter de-sensitizing toothpaste that prevents the transmission of nerve endings to the teeth Its chemical formula is KNO3. Crystalline (sand-like) powder or solid with a sharp, salty taste.
HOW DO WE USE POTASSIUM NITRATE Potassium nitrate is used for pesticides, glass, fireworks, explosives, and rocket fuels. It is also used as a food preservative, and when added to meat it causes a reaction between the myoglobin and hemoglobin in the blood, making the meat appear red in the colour. It has also been used in the manufacture of ice cream and can be found in some toothpastes for sensitive teeth. It is used for carefully melting together using a hot plate. Potassium nitrate, also known as saltpeter. It is a crystalline salt that is composed of potassium ions (K+) and nitrate ions (NO3 ).