Evolution of Antiretroviral Therapy: Dual vs. Triple ART
The evolution of antiretroviral therapy (ART) from dual to triple drug regimens has been crucial in improving outcomes for HIV patients. Research findings demonstrate the superiority of three-drug therapy over two-drug therapy in terms of efficacy and resistance management. Maintenance ART strategies also play a significant role in managing virologic suppression. Studies comparing different drug regimens help guide healthcare providers in choosing the most effective treatment approach for HIV patients.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Dual vs. Triple ART: Dual vs. Triple ART: What to start? What to start? R.M. Gulick, MD, MPH Professor of Medicine Weill Cornell Medicine New York City
Disclosures Disclosures none
Evolution of ART (1 Evolution of ART (1 1994: 2-drug NRTI therapy superior to monotherapy ACTG 175 (N=2467): Hammer NEJM 1996;335:1081-90 Delta (N=3308): Delta Study group Lancet 1996;348:283-91 CPCRA (N=1113): Saravolatz NEJM 1996;335:1099-106 NUCA 3001 (N=366): Eron NEJM 1995;333:1662-9 NUCB 3001 (N=223): Staszewski JAMA 1996;276:111-117 2 2 3 drugs) 3 drugs) 1996: 3-drug therapy with 2 NRTI + PI (or NNRTI) superior to 2 NRTI ACTG 320 (N=1156): Hammer NEJM 1997;337:725-33 MRK 035 (N=97): Gulick NEJM 1997;337:734-9 INCAS (N=153): Montaner JAMA JAMA 1998;279:930-7 number of drugs: toxicity, complexity, class-sparing, cost
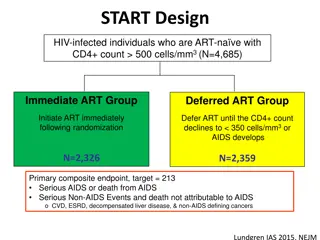
Maintenance ART: 2 Maintenance ART: 2- -Drug vs. 3 Drug vs. 3- -Drug Drug ACTG 343 (N=509) ZDV/3TC/IDV ZDV/3TC or IDV or continue 3 drugs Trilege (N=279) ZDV/3TC/IDV ZDV/3TC or ZDV/IDV or continue 3 drugs (stopped early) ADAM (N=62) d4T/3TC/SQV/NFV d4T/NFV or SQV/NFV or continue 4 drugs (stopped early) Cochrane Systematic Review: Loss of virologic suppression Havlir NEJM 1998;339:1261 1268 Flandre AIDS 2002;16:561-8 Reijers Lancet 1998;352:185 190 Rutherford Cochrane Rvw 2003;4:CD002037
What to Start?: 2 What to Start?: 2- -Drug Regimens (1) PI/r + NNRTI (NRTI-sparing) ACTG 5142 (2 NRTIs with [EFV or LPV/r] vs. LPV/r + EFV) Study population: treatment-na ve; HIV RNA >2000; resistance testing if HIV-infected <1 year (N=757) Drug Regimens (1) 89% 2 NRTIs + EFV 83% LPV/r + EFV 77% 2 NRTIs + LPV/r LPV/r + EFV: resistance at virologic failure and lipids Riddler NEJM 2008;358:2095
What to Start?: 2 What to Start?: 2- -Drug Regimens (2) PI/r + TDF KALEAD (N=152) LPV/r + TDF (vs. LPV/r + 2 NRTI) >40% discontinuation of study meds; TDF not non-inferior PI + integrase inhibitor SPARTAN (N=94) ATV + RAL (vs. TDF/FTC + ATV/r) stopped early 20% grade 4 hyperbilirubinemia; virologic failure + RAL resistance ACTG 5262 (N=112) DRV/r + RAL single-arm study 26% with virologic failure at week 48 Drug Regimens (2) Pinola J Antivir Antiretrovir 2010;2:56-62 Kozal HIV Clin Trials 2012;13:119-130 Taiwo AIDS 2011;25:2113
What to Start?: 2 What to Start?: 2- -Drug Regimens (3) PI/r + 3TC GARDEL (LPV/r + 3TC vs. 2 NRTI + LPV/r) Study population: Rx-naive, HIV RNA >1000, no chronic HBV infection, no resistance to NRTIs, LPV, RTV (N=426) Drug Regimens (3) 88% 2-drug ART 84% 3-drug ART (with more tox d/c) ( +4.6%, 95% CI: 2.2%, +11.8%; p=0 171) 2-drugs non-inferior 54% on 3-drugs on ZDV Cahn Lancet Infect Dis 2014;14:572
What to Start?: 2 What to Start?: 2- -Drug Regimens (4) PI/r + integrase inhibitor PROGRESS: (N=206) LPV/r + RAL (vs. TDF/FTC + LPV/r) <70% virologic suppression overall at week 96 NEAT-001: (N=805) DRV/r + RAL (vs. TDF/FTC + DRV/r) Subgroup analysis: DRV/r + RAL inferior if CD4 <200, VL >100,000 Drug Regimens (4) Reynes AIDS Res Hum Retro 2013;29:256 Raffi Lancet 2014;384:1942 PI/r + CCR5 antagonist MODERN (N=797) DRV/r + MVC (vs. TDF/FTC + DRV/r) DSMB stopped study early due to lack of efficacy in MVC arm Stellbrink AIDS 2016;30:1229
Meta Meta- -Analysis: 2 Analysis: 2- -drug Initial ART Regimens (2008 drug Initial ART Regimens (2008- -15) 15) Outcome: Virologic Failure (N=11) For baseline HIV RNA >100,000: RR 1.24 (95% CI: 1.03, 1.49) Achhra Lancet HIV 2016;3:e351-e360. For resistance mutations: RR 2.04 (95% CI: 1.23, 3.39)
What to Start?: 2 What to Start?: 2- -Drug Regimens (6) DTG + 3TC PADDLE Study Cahn JIAS 2017;20:1-7; Figueroa IAS 2017 #MOPEB0287 Treatment-na ve individuals with HIV RNA 5-100K (N=20) Results: All suppressed VL <50 by week 8 18/20 (90%) remained suppressed through week 96 1 had VL 99 246 61 with no RT mutations, then resuppressed 1 had adverse event (suicide) between weeks 24 and 36 Drug Regimens (6) ACTG 5353 Treatment-na ve, HIV RNA up to 500K (N=120) 90% <50 copies/ml at week 24 (FDA snapshot analysis) One pt with suboptimal adherence developed 2-class resistance Taiwo CID 2018;66:1689
What to Start?: 2 What to Start?: 2- -Drug Regimens (7) Drug Regimens (7) DTG + 3TC GEMINI 1 and 2 (N=1441) Randomized, double-blinded, international phase 3 study Study population: Rx-naive, HIV RNA 1000-500,000, no chronic HBV infection, no major resistance mutations Cahn Lancet 2019;393:143-155 93% TDF/FTC + DTG 91% DTG + 3TC -1.7 (95% CI: -4.4, +1.1) 2 drugs non-inferior no resistance April 2019 96 week results: Cahn IAS 2019 #WEAB0404LB
Emerging 2 Emerging 2- -drug ART Regimens drug ART Regimens DRV/r + 3TC ANDES (N=182): DRV/r + 3TC (vs. TDF/3TC + DRV/r) open-label 93% with HIV RNA <50 on 2-drug ART non-inferior to 3-drug ART Figueroa CROI 2018 #489 DRV/r + DTG D2EFT (N=1010): second-line therapy regimens DRV/r + DTG (vs. 2 NRTI + DRV/r vs. 2 NRTI + DTG) clinicaltrials.gov #NCT03017872 islatravir (MK-8591) + DOR Molina IAS 2019 #WEAB0402LB
ART Guidelines: What to Start? 2-drug ART: Alternative or Other Guideline 2-drug ART scenario US DHHS 2018 www.aidsinfo.nih.gov TDF cannot be used or are not optimal regimens DRV/r + RAL* DRV/r + 3TC DTG + 3TC DRV/c or /r + RAL or DTG or 3TC or FTC DRV/c or /r + RAL* DTG + 3TC none when ABC, TAF, and IAS-USA 2018 JAMA 2018;320:379 when individuals cannot take ABC, TAF, or TDF EACS 2018 www.europeanaidsclinical society.org/ WHO 2018 http://www.who.int/hiv/pub/guid elines/en/ alternative none * performs less well/not recommended for baseline HIV RNA >100,000 or CD4 <200
Switching: Maintenance 2 Switching: Maintenance 2- -Drug ART Regimens (1) Drug ART Regimens (1) PI/r + 3TC (vs. 2 NRTIs + PI/r) OLE (LPV/r, N=250) ATLAS-M (ATV/r, N=266) SALT (ATV/r, N=286) DUAL (DRV/r, N=249) Arribas Lancet Infect Dis 2015;15:785 Fabbiani JIAS 2014;17:19808 Perez-Molina Lancet ID 2015;15:775 Pulido CID 2017;65;2112 DTG + 3TC ASPIRE: (N=90) LAMIDOL/ANRS 167: (N=110) TANGO: (N=700) Taiwo Clin Infect Dis 2018;66:1794 7 Joly JAC 2019;74:739-745 Van Wyk IAS 2019 #WEAB0403LB
Switching: Maintenance 2 Switching: Maintenance 2- -Drug ART Regimens (2) II + NNRTI SWORD 1 and 2 (DTG/RPV; N=1028) Drug ART Regimens (2) Llibre Lancet 2018;391:839 November 2017 May 2018 LATTE 2 (CAB + RPV; N=309) ATLAS (CAB + RPV; N=616) FLAIR (CAB + RPV; N=629) DRV/r + DTG TIVISTA (Italian Cohort; N=113) Spanish Cohort (N=50) DUALIS (N=263) HIV RNA <50: 86% (2 drugs) vs. 88% (3 drugs) Margolis Lancet 2017;390:1499 Swindells CROI 2019 #139 Orkin CROI 2019 #140 Capetti Antivir Ther 2017;22:257-262 Navarro Pharmacother 2019;39:501-507 Spinner IAS 2019 #MOPEB269
2 2- -Drug Initial ART: Conclusions Drug Initial ART: Conclusions 2-drug ART regimens challenge current 3-drug ART regimens. Optimal 2-drug ART regimens are potent, convenient, well-tolerated, have a high barrier to resistance, and are drug-sparing. Strongest 2-drug initial ART data: Boosted PI + 3TC Caveats: baseline resistance testing, HBV co-infection, side effects/drug interactions DTG + 3TC Caveats: VL <500K, baseline resistance testing, HBV co-infection, pregnancy, 2-class drug resistance (rare), durability Mixed 2-drug initial ART data: Boosted PI + INSTI Emerging 2-drug regimens: DRV/r + 3TC, DRV/r + DTG, others Current guidelines recommend 2-drug regimens as alternative/other this may change!
Acknowledgments Acknowledgments Cornell HIV Clinical Trials Unit (CCTU) Division of Infectious Diseases Weill Cornell Medicine AIDS Clinical Trials Group (ACTG) HIV Prevention Trials Network (HPTN) Division of AIDS, NIAID, NIH Industry partners The participant volunteers!