Energy Basics: Temperature, Heat, and Transfer
Explore the fundamental concepts of energy, including temperature, heat, and energy transfer. Learn how temperature differs from heat, how they are measured, and the relationship between thermal energy and water volume. Discover the role of chemical energy and sound energy, and how energy is transformed and transferred in various ways, such as to light and kinetic energy. Gain insights into the Law of Conservation of Energy.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Energy basics Energy basics
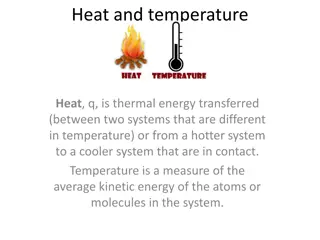
Temperature Temperature and Heat and Heat Temperature and heat are NOT THE SAME.
Temperature Temperature How hot or cold it is. Measured in degrees Celsius degrees Celsius.
Heat Heat The amount of thermal energy, measured in joules or J joules or J. A cup of hot tea has heat energy heat energy in the form of kinetic energy energy from its particles. kinetic
A swimming pool at 30C is at a lower temperature than a cup of tea at 80 C. BUT the swimming pool contains more water, so it stores more thermal energy or heat.
The small beaker of The small beaker of water boils first water boils first The large beaker contains more water and needs more thermal energy or heat to reach 100 C.
Chemical energy Chemical energy stored in it is transferred to the surroundings as thermal energy thermal energy, sound energy energy and kinetic energy kinetic energy. sound
Sound energy Sound energy can be transferred to your eardrum as kinetic energy kinetic energy (movement energy).
The battery transfers stored chemical chemical energy energy as electrical energy energy. The electrical energy is transferred to the surroundings by the lamp as light energy light energy and thermal energy thermal energy (heat energy). electrical
Energy Energy transfer transfer
The Law of Conservation of Energy Energy is always conserved, it is never "lost" or "wasted . Some energy transfers are useful and some are not.
Sankey diagrams Sankey diagrams