Current Trends in Drug Pricing Legislation and Medicare Reimbursement Updates
Democrats currently control the agenda with proposed principles for drug pricing reform, while Republicans eye upcoming midterms. Understanding potential shifts in control and how they could impact drug pricing, negotiation authority, and market reforms is crucial. Additionally, an update on Medicare Part B reimbursement and the return of sequestration effects are highlighted.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Whats Happening on the Hill: Insights on What s Happening on the Hill: Insights on the Latest Drug Pricing & Reporting the Latest Drug Pricing & Reporting Proposals Proposals Andrew Ruskin Partner K&L Gates LLP (202) 778-9415 Andrew.ruskin@klgates.com Stephanie Trunk Partner ArentFox Schiff LLP (202) 857-6171 stephanie.trunk@afslaw.com
Democrats currently control the agenda Democrats currently control the agenda Sen. Wyden (D), Chair of the Senate Finance Committee, published his Principles for Drug Pricing Reform in June, 2021 These principles include: Medicare needs negotiation authority for drug pricing, which currently would violate law Patients need to pay less at the pharmacy OIG s PBM rule viewed as counterproductive currently on hold for 3 years Drug costs cannot increase faster than inflation All Americans, not just Medicare enrollees, should benefit from pricing reform Patent reform is needed
But the midterms are coming . . . But the midterms are coming . . . The Republicans only need one seat in the Senate to have a majority (and Sen. Manchin would no longer control the agenda) The House currently has 209 Republicans and 221 Democrats, but, by some estimates, 26 seats are a toss-up
What if the Republicans take over? What if the Republicans take over? They are less likely to impose price controls, but still favor government negotiations and other market reforms They also favor price transparency, such as use of tools to compare receiving drugs in different settings Essentially, while more modest, there would still be room for compromise on legislation that ultimately gets passed into law
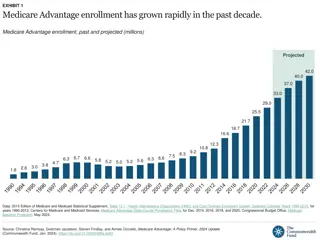
Medicare Part B Reimbursement Medicare Part B Reimbursement
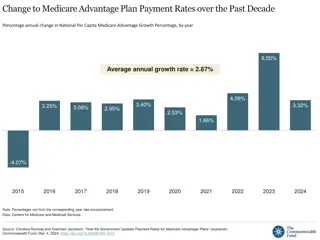
Sequestration Sequestration- - It s Back! It s Back! Medicare payments, including for buy and bill drugs paid for by Medicare Part B, were subject to 2% sequestration This resulted in reimbursement for separately payable drugs being reduced to Average Sales Price +4.3% as opposed to +6% Sequestration suspended through March 2022 but phasing back in first 1% cut on 4/1/22, second 1% cut is slated to go into effect on 7/1/22 under the Protecting Medicare and American Farmers from Sequester Cuts Act, which became law in December 2021
Wastage Rebate Wastage Rebate Included in Infrastructure Investment and Jobs Act signed into law Nov. 2021 (P.L. 117-58) First rebate quarter is Q1 2023 Requires refund/rebate of portion of reimbursement to DHHS for certain discarded single-dose or single-use drugs Rebate determined by total amount of discarded medication above a 10% threshold and billed based on the discarded volume in claims Claims identified with JW modifier by administering physicians
Build Back Better Act Build Back Better Act H.R. 5376 passed the House on Nov. 19, 2021 HHS to negotiate on drug prices Applicable to Part B starting 2027 HHS to select top 50 drugs with highest total Part B spending Then allowed to negotiate price on a subset 15 drugs in 2027 20 drugs in 2028 and later
Build Back Better Act Build Back Better Act Inflation rebate Effective 2023 Rebate owed if single-source drugs and biologicals covered under Part B take price increases faster than inflation Looks at changes to average sales price Rebate amount = total number of units * amount the price exceeds the inflation adjusted payment amount [including ALL units sold outside of Medicaid] Base year used to measure cumulative price changes is 2021 Steep penalty for not paying rebate = 125% of original rebate amount
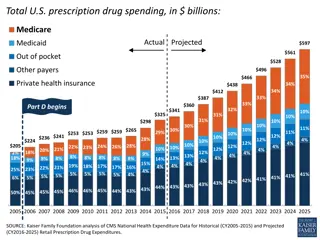
Medicare Part D Reimbursement Medicare Part D Reimbursement
Build Back Better Act Build Back Better Act HHS to negotiate on drug prices Allows for HHS price negotiation via amendment to non-interference clause Applicable to Part D starting 2025 HHS to select top 50 drugs with highest total Part D spending and all insulin products Then allowed to negotiate price on a subset 10 drugs in 2025
Build Back Better Act Build Back Better Act Inflation rebate Effective 2023 Applies to nearly all drugs covered by Part D Based on changes to AMP Rebate amount = total number of units * amount the price exceeds the inflation adjusted payment amount [including ALL units sold outside of Medicaid] Base year used to measure cumulative price changes is 2021 Steep penalty for not paying rebate = 125% of original rebate amount
Build Back Better Act Build Back Better Act Benefit design changes Hard cap on beneficiary out-of-pocket spending - $2,000 in 2024 Will increase each year based on rate of increase in per capita Part D costs Caps insulin copays at $35/mo Lowers beneficiary share of total drug costs for initial coverage phase Manufacturers will have increased responsibility for brands/biologics/biosimilars during initial coverage phase = 10% of costs 0% for generics Will finally eliminate the coverage gap Manufacturers will have 20% responsibility of costs for brands/biologics/biosimilars during the catastrophic coverage phase 0% for generics
Build Back Better Act Build Back Better Act
Build Back Better Act Build Back Better Act- - The Heat is On The Heat is On In April 2022, Organizations like AARP and Center for American Progress have been pressing Democrats to pass some sort of drug pricing legislation that might let CMS negotiate directly with manufacturers for rebates for Medicare Senator Wyden has been meeting with Senator Manchen to try to move a drug pricing bill forward. Wyden tells the press we re talking.
Medicaid Medicaid
MDRP Cap on Rebates Eliminated MDRP Cap on Rebates Eliminated Under the American Rescue Plan Act, MDRP rebates no longer capped at AMP Manufacturers under water on certain covered outpatient drugs leading to discontinuance, shortage? Manufacturers discontinuing participation in Medicaid? Beneficiaries accessing fewer drugs? Effective date January 1, 2024
Inclusion of Manufacturer Co Inclusion of Manufacturer Co- -payment Assistance in Best Price Assistance in Best Price MDRP Rule published December 21, 2020 payment Excludes manufacturer coupon or co-pay cards from Medicaid metrics if used by individuals enrolled in a health plan with a co-pay accumulator program (doesn t count toward out of pocket costs) January 1, 2023. Manufacturers may curtail co-payment assistance or got to old school paper rebates after purchase; also talk of manufacturers perhaps offering a secondary insurance type product Per Drug Channels- based on MMIT data- For 2021, 80% of commercially insured beneficiaries are enrolled in plans with copay accumulators available in the plan design, while 61% are enrolled in plans with maximizers in the plan design
Legislation proposed to curtail Legislation proposed to curtail accumulators accumulators HR 5801 (bipartisan) Introduced on 11/1/21 must count towards OOP States that any amounts paid on behalf of a patient in a group health plan for qualified health plans on an exchange Would also ensure that these amounts count towards patient cost sharing PhRMA suit ongoing
Gene Therapy BP Exception Gene Therapy BP Exception Part of the Lower Cost, More Cures Act of 2021 proposed legislation Introduced by Sen. Crapo (R) Would allow for refunds and delayed payments for gene therapies drugs, when part of an outcomes-based agreement, without consequences under BP Would require that the States be offered the opportunity to enter into such an agreement
340B Saga 340B Saga
The Problem is the Statute The Problem is the Statute The statute requires only that the manufacturer shall . . . offer covered entities covered outpatient drugs for purchase at the 340B price. There is no reference to: Any ability or lack of ability to attach strings to those purchases Any limitation or lack of limitation on locations where the covered entity can use covered outpatient drugs The statute requires that covered entities use the drugs only with patients, but there is no definition of patient in the statute The statute gives HRSA the authority to issue regulations only in connection with the assessment of penalties against manufacturers, and in creating an alternative dispute resolution process It does not give HRSA the ability to issue regulations to interpret substantive areas of law Most courts have noted that their hands are tied, without further legislation
340B Saga 340B Saga Current status of manufacturer litigation AstraZeneca Pharms. LP v. Becerra (U.S. District Court for the District of Delaware) (Case 1:21-cv-00027) Invalidated Advisory Opinion; recently ruled that HRSA letter is vacated and set aside Most recent action; HRSA appealed to 3rd circuit 4/12/2022 Eli Lilly & Co. v. U.S. Dep t of Health & Human Servs. (U.S. District Court for the Southern District of Indiana) (Case 1:21-cv-00081) Enjoined ADR rule in this case only Invalidated Advisory Opinion and remanded enforcement consideration to HRSA On appeal in 7th Circuit- cross appeals
340B Saga 340B Saga Current status of manufacturer litigation Sanofi-Aventis U.S., LLC and Novo Nordisk Inc. v. Dep t of Health & Human Servs. (U.S. District Court for the District of New Jersey) (Cases 3:21-cv- 00634 and 3:21-cv-00806) Upheld HRSA authority to require recognition of contract pharmacies and upheld ADR rule Remanded enforcement consideration to HRSA On appeal in 3rd Circuit- cross appeals Novartis Pharms. Corp. and United Therapeutics v. Espinosa (U.S. District Court for the District of Columbia) (Cases 1:21-cv-01479 and 1:21-cv-01686) Manufactures can impose some restrictions- unclear at to which; vacated enforcement action/letters On appeal in D.C. Circuit- HRSA filed only
340B Saga 340B Saga 16 manufacturers are not recognizing/limiting recognition of contract pharmacies including AbbVie, UCB and Amgen of lately HRSA has turned over 7 to VA OIG for potential CMP enforcement ADR panel is hearing disputes- 340B Administrative Dispute Resolution (ADR) | Official web site of the U.S. Health Resources & Services Administration (hrsa.gov) Must be $25,000 or more in dispute over a 12-month period
Federal Transparency Efforts Federal Transparency Efforts
Hospital Disclosure Rule Hospital Disclosure Rule ACA authorized DHHS to issue regulations compelling hospitals to disclose relevant prices. Rulemaking put law into effect in 2015 with an update that became effective January 2021. These prices must be posted online in formats that allow third-party technology companies to build price comparison tools. All hospitals must post 5 separate prices for their billing codes the full chargemaster, which is the list price before any discounts the discounted cash price paid by self-paying individuals the in-network negotiated prices for each insurance plan with which a hospital has a contract the lowest in-network price charged (with identification of the insurer hidden) the highest in-network rate (also with the insurer hidden from view). Non-compliance penalty recently raised from $109,500 annually to nearly $2 million per year beginning in 2022.
Plan/PBM Disclosure Rule Plan/PBM Disclosure Rule Rulemaking effective January 2022; delayed until July 1, 2022 with additional reporting January 1, 2023 Health plans, including those sponsored by self-insured employers, must post online for public consumption their in-network prices, their out-of-network allowed charges, prices for 500 items/services Insurers must build and maintain a consumer pricing tool for their enrollees that allows for real-time estimates of expected out-of- pocket costs for scores of potential services.
Plan/PBM Disclosure Rule Plan/PBM Disclosure Rule Requires health plan to discloses if there is a co-payment coupon accumulator or maximizer program (when a health plan doesn t count manufacturer coupons to out of pocket maximums) PCMA and U.S. Chamber of Commerce had challenged the prescription drug transparency requirements but withdrew lawsuit when rulemaking required reporting of net prescription drug prices to federal government but agreed not to make public First reporting December 27, 2022
STAR Act STAR Act Sunshine, Transparency, Accountability and Reporting Act ( STAR ) was a bipartisan effort in 2019-2020 for federal drug transparency reporting aimed at manufacturers- did not pass into law Would require manufacturers to justify/explain prices increases of 10% or $10,000 per year or 25% or $25,000 in 3 years; PBM reporting of discounts/rebates; DHHS study on inpatient drug costs Could have been welcome by some manufacturers to preempt state reporting
Qualified Health Plans Qualified Health Plans- - Exchanges Exchanges
Qualified Health Plans Qualified Health Plans American Rescue Plan Act became law on March 11, 2021. It expanded the premium tax subsidies for individuals enrolled in Quailed Health Plans sold on Exchanges. Anyone is entitled to the subsidies rather than just those at or below 400% of the Federal Poverty level. It also increased subsidies for those at or below 400% of the Federal Poverty level. Without Congressional action, the expansion of the subsidies expires the end of 2023, which could result in millions of Americans forgoing coverage
Other Items of Note Other Items of Note
Buy American Preference Buy American Preference Biden Administration issued Executive Order to strengthen Buy American preferences on Jan. 25, 2021 Extends to pharmaceuticals Federal government agencies not permitted to waive Buy America Act/Trade Agreements Act requirements Expect to see more federal grants incentivizing production of pharmaceuticals in the United States/stockpiles of U.S. made drugs- opportunity for wholesale distributors
Stockpile Act Stockpile Act Essential Medicines Strategic Stockpile Act of 2021 (H.R. 2870) Introduced to House April 2021; no vote so far Would create an additional stockpile of medicines Generic drugs at risk of shortage - drugs approved under ANDAs on the World Health Organization Model List of Essential Medicines and on FDA shortage list at some time in last 36 months or needed for public health emergency 3-6 month stockpile held by manufacturers/wholesalers/distributors- purchased inventory but paid a fee by the federal government to carry a certain level of inventory
Importation Importation Trump Administration published a final rule and FDA guidance permitting states to import drugs from Canada and other countries; was effective November 30, 2020 On November 23, 2020, Pharmaceutical Research and Manufacturers of America ( PhRMA ), Partnership for Safe Medicines ( PSM ), and Council for Affordable Health Coverage ( CAHC ) filed a lawsuit challenging the Final Rule. Department of Justice filed a motion to dismiss on May 28, 2021 Court has not yet ruled on motion to dismiss. A few days after the case was filed, Canada passed an interim order banning the export of certain drugs from Canada.
Importation Importation In July 2021, President Biden issued an Executive Order on Promoting Competition in the American Economy that directs the FDA to work with States and Indian Tribes that propose to develop Section 804 Importation Programs. FDA updated its website on Human Drug Imports website for states and tribes interested in developing a Section 804 Importation Program, including contact information, to facilitate engagement between the agency and states and tribes, in August 2021. Several states have submitted proposals- Florida, Vermont, Colorado, New Mexico and Maine.
Importation Importation In April 2022, FDA met with 5 states- Florida, Colorado, Vermont, Maine and Mew Mexico to discuss how to create their proposed Canadian drug importation programs. An FDA spokesperson told reports The FDA is committed to working with states and Indian tribes that propose to develop importation programs to reduce the cost of products to the American consumer while still protecting public health and safety.
Questions? Questions? Andrew Ruskin Partner K&L Gates LLP (202) 778-9415 Andrew.ruskin@klgates.com Stephanie Trunk Partner ArentFox Schiff LLP (202) 857-6171 Stephanie.Trunk@afslaw.com